Episode 238: Creatine and Mental Health
Joshua Mangunsong, Liam Browning, Brandon Luu, MD; Nicholas Fabiano, MD; David Puder, MD
Corresponding author: David Puder, MD
Reviewer: Erica Vega, Joanie Burns, PMHNP-BC
None of the authors/presenters have any conflicts of interest.
By listening to this episode, you can earn 1.5 Psychiatry CME Credits.
Abstract:
Creatine, widely known for enhancing athletic performance, is gaining attention for its potential as a novel adjunct psychiatric treatment. Beyond muscle function, creatine supports brain energy metabolism, which has increasing evidence for a role in mood disorders like depression and bipolar disorder.
Recent studies suggest creatine supplementation can enhance treatment outcomes in psychiatric conditions. Sherpa et al. (2025) found that adding creatine to cognitive behavioral therapy (CBT) significantly improved depressive symptoms. Similarly, Lyoo et al. (2012) reported creatine enhanced response to SSRIs in women with major depressive disorder.
Neuroimaging studies indicate creatine boosts phosphocreatine levels in brain regions linked to mood regulation, and doses above the normal 5 grams per day have added increases in brain creatine levels. Observational data also suggest an inverse relationship between dietary creatine intake and depression prevalence.
This article and accompanying podcast episode provides a scoping review of the evidence on creatine’s psychiatric applications, exploring its mechanisms, clinical relevance, and future research directions.
Introduction
Creatine is a naturally occurring compound essential for cellular energy metabolism, primarily through its role in adenosine triphosphate (ATP) regeneration via the phosphocreatine system (Miller, 2022). It is widely recognized for its benefits in sports performance, muscle growth, and recovery, as well as its potential neuroprotective properties. Beyond its established role in muscle physiology, emerging evidence suggests creatine may have significant effects on brain bioenergetics, cognition, and mental health (Prokopidis et al., 2023). There are several mechanisms that may explain these effects.
Animal models offer additional support for creatine’s neurotherapeutic potential. In a study examining the impact of dietary creatine on experimental traumatic brain injury in rats, supplementation with a diet containing 1% creatine for four weeks significantly reduced cortical damage by 50% (p < 0.01). Given typical daily food consumption in rats, this 1% dietary supplementation approximates a daily intake of around 0.5–1 g/kg, translating to approximately 35–70 grams of creatine per day—for a 70 kg human. The observed neuroprotective effects are thought to arise from enhanced mitochondrial bioenergetics, characterized by increased mitochondrial membrane potential, reduced calcium accumulation, lower reactive oxygen species production, sustained ATP levels, and inhibited opening of mitochondrial permeability transition pores (MPTP) (Sullivan et al., 2000). Another study found that high-dose creatine supplementation (approximately equivalent to 280 g/day in a 70 kg human, significantly higher than typical human dosages) attenuated striatal dopamine depletion in a mouse model of Parkinson’s disease, reducing dopamine loss from 56% in untreated mice to 33% in creatine-treated mice (p < 0.05) (Yang et al., 2009). In a mouse model of chronic mild stress-induced depression, creatine supplementation significantly reduced depressive behaviors measured by the forced swim test, decreasing immobility time from 106.7 seconds in stressed controls to 39.0 seconds with creatine alone, and further down to 34.1 seconds with combined creatine plus exercise treatment. Serotonin (5-HT)-positive neurons in the dorsal raphe nucleus increased substantially from 55.0 cells in stressed controls to 126.8 cells with creatine alone, and even more markedly to 206.1 cells with the combination of creatine and exercise. The creatine dose used (~4% dietary inclusion) translates to approximately 140 grams per day for a 70 kg human, far exceeding the standard human supplementation dose of 5 grams per day, indicating results achieved at a high pharmacological dose (Ahn et al., 2016).
In humans, a study found that creatine supplementation as an add-on to cognitive-behavioral therapy (CBT) demonstrated potential in treating depression, as evidenced by a greater reduction in Patient Health Questionnaire-9 (PHQ-9) scores compared to CBT with placebo (Sherpa et al., 2025).
Emerging neurobiological research has highlighted creatine’s role in brain bioenergetics, with supplementation shown to increase brain phosphocreatine levels and enhance cognitive processes such as memory, attention, and information processing speed (Avgerinos et al., 2018; Xu et al., 2024). Meta-analyses have found more pronounced effects in older adults and under conditions of metabolic stress, such as sleep deprivation, although evidence for broader executive function remains inconclusive (Avgerinos et al., 2018, Prokopidis et al., 2023). These findings suggest creatine’s utility may extend beyond its known ergogenic effects into domains of cognitive and mental health.
Mental health disorders are a leading cause of disability worldwide. In 2019, approximately 1 in every 8 people, or 970 million individuals globally, were living with a mental disorder, with anxiety and depressive disorders being the most common (World Health Organization, 2022). Conventional treatments, including pharmacotherapy and psychotherapy, are effective for many but often have limitations such as delayed onset, incomplete response, and adverse side effects (Hoskins et al., 2015; Howes et al., 2022; Leichsenring et al., 2022). The growing field of nutritional psychiatry emphasizes the role of diet and supplementation in mental health, leading to increasing interest in creatine due to its safety profile, accessibility, and emerging evidence supporting its use in psychiatric treatment.
Concerns about creatine’s long-term renal safety have been common, largely due to elevated serum creatinine levels, which can be misinterpreted as signs of kidney dysfunction. However, a frequently cited study done in 1999 followed nine healthy athletes who had used creatine for 10 months to 5 years at doses ranging from 1 to 20 g/day (Poortmans & Francaux, 1999). Compared to 85 non-supplementing controls, no significant differences were found in serum creatinine, BUN, creatinine clearance, or urinary creatinine excretion. These findings support the renal safety of prolonged creatine use in healthy individuals, and remain one of the most cited pieces of real-world safety data.
While several systematic reviews have investigated creatine’s effects on cognition and neurological function, few have comprehensively addressed its psychiatric applications. Existing reviews often focus on general populations or specific cognitive outcomes, leaving gaps in understanding its role across diverse psychiatric disorders (Avgerinos et al., 2018; Xu et al., 2024). Despite promising preliminary findings, research on creatine’s psychiatric applications remains in its early stages. Many studies focus on specific diagnoses, often with small sample sizes and inconsistent methodologies, leading to mixed findings (Juneja et al., 2024). Additionally, while the potential mechanisms of creatine’s effects on mood and cognition are being explored, they remain incompletely understood. Notably, no comprehensive synthesis has systematically examined creatine’s role across multiple psychiatric disorders, leaving a critical gap in the literature.
This scoping review aims to systematically map the existing literature on creatine supplementation across various psychiatric disorders. By synthesizing findings from diverse studies, this review seeks to provide a clearer picture of creatine’s potential clinical applications, effectiveness, and underlying mechanisms. Identifying knowledge gaps will also help guide future research directions and inform potential clinical recommendations.
The primary objective of this review is to explore and summarize current knowledge on creatine supplementation in relation to different psychiatric conditions. By assessing the breadth of existing evidence, this review will help determine the extent of creatine’s potential benefits for mental health and highlight areas where further investigation is needed. Before we begin the review, we will first introduce creatine’s potential mechanism for mood disorders and also prior studies on sleep as they add to the overall understanding of prior research.
Creatine’s Potential Mechanism For Mood Disorders
With regard to its mechanism, creatine is most well-known for its role in the creatine/phosphocreatine (Cr/CrP) buffer system. Phosphocreatine can transfer a phosphate group to ADP in order to regenerate ATP during periods of high energy demand (Adhihetty & Beal, 2008). While glycolysis and oxidative phosphorylation can take between 30 seconds and a few minutes to replenish ATP, the Cr/CrP system can regenerate ATP in as little as 5–10 seconds. In exercising muscle, this ATP supplied by phosphocreatine is often what allows someone to perform an extra rep or two during sets.
Muscle cells typically rely first on aerobic respiration, fueled by pyruvate entering the TCA cycle and by fat oxidation. When energy demands escalate and less oxygen is available, anaerobic respiration increases, burning glucose for short-term fuel but yielding less ATP and producing lactic acid (leading to the “burn” felt in muscles). Because anaerobic pathways take up to 30 seconds to 2 minutes to recover, the Cr/CrP system steps in to supply rapid energy for approximately 5–10 seconds until muscle exhaustion (Mahoney et al., 2002).
Additionally, the Cr/CrP system buffers intracellular ATP levels, improving mitochondrial function. One study by Walsh et al. (2001) showed creatine increased mitochondrial ATP production by 60% in muscle tissue during intense exercise. The authors suggest that excess ATP (and thus limited ADP availability) within the cell inhibits key metabolic enzymes, signaling that no further glucose or fat breakdown is needed. This can lead to an accumulation of substrates and metabolic intermediates, some of which may be toxic at high concentrations and reduce mitochondrial efficiency. A buildup of ATP can also slow the electron transport chain, potentially increasing the leakage of electrons and the formation of reactive oxygen species (ROS), known to damage cells. Several studies in mouse models of neurodegenerative diseases (e.g., ALS, Huntington’s disease, Parkinson’s disease) suggest creatine’s ROS-reducing properties and mitochondrial membrane stabilization capabilities may improve neuronal survival, motor function, and overall survival (Dedeoglu et al., 2003). However, these benefits have not consistently translated to humans (Forbes et al., 2022).
To recognize creatine’s potential role in neurons, it is important to understand how neurons generate and use ATP. Neurons constantly consume energy, with the brain using around 20% of the body’s calories despite being only ~2% of its mass (Attwell & Laughlin, 2001). However, unlike muscle cells, neurons do not uptake a significant amount of glucose or fat, meaning their glycolytic and fat oxidation capacities are limited. Instead, neurons mainly rely on aerobic respiration with lactate serving as the primary fuel source via the astrocyte-neuron lactate shuttle. Astrocytes take in glucose from the blood, perform glycolysis to generate lactate, and shuttle this lactate to neurons; the neurons then convert lactate into pyruvate for use in the TCA cycle (Mason, 2017). Nevertheless, because some neuronal processes require energy on the order of milliseconds, the Cr/CrP system in the brain helps meet these immediate energy demands. Examples of some of the most time-sensitive, energetically demanding processes at the cellular level include the Na⁺/K⁺-ATPase pump (regulates resting membrane potential), synaptic vesicle release and recycling, and calcium regulation through Ca²⁺-ATP pumps. Burst-firing neurons—such as CA3 neurons in the hippocampus, thalamic relay neurons, cortical pyramidal cells, Purkinje neurons in the cerebellum, and dopaminergic neurons in the substantia nigra and ventral tegmental area—have high energy demands and are particularly susceptible to energy deficits (Joo et al., 2021; Krahe & Gabbiani, 2004). Other energetically demanding states also include sleep deprivation, hypoxia, aging, and depression.
It should be noted that the brain is one of the few organs (alongside the liver, kidneys, pancreas) that endogenously produces creatine, likely owing to the time-sensitive and energetically demanding tasks mentioned above. However, because of this endogenous production, brain cells store significantly less creatine than muscle tissue and have limited ability to transport creatine across the blood-brain barrier, which is cause for debate about creatine supplementation’s ability to increase brain creatine levels (Forbes et al., 2022). Nevertheless, studies in which participants are placed under energetically demanding states or have lower brain creatine at baseline consistently show increased brain creatine uptake and improvements in cognitive function (Forbes et al., 2022).
Based on the neuroenergetics hypothesis, one proposed antidepressant mechanism is that exogenous creatine, by bolstering ATP availability, may facilitate optimal neuronal firing for complex cognitive processes, especially in patients with limited brain creatine stores or who are engaging in intensive cognitive tasks, such as therapy. Higher cognitive processes, including complex meta-conscious activities (awareness of the contents of one’s consciousness) and insight formation, place significantly greater energetic demands on the brain than do simple sensory processing or brainstem functions (Chen & Zhang, 2021). These higher-order brain activities require more extensive spatiotemporal coordination among neurons in disparate brain regions, often manifesting through synchronized gamma oscillations (40–100 Hz), which are energetically costly and rely on robust ATP availability (Joo et al., 2021). Consequently, maintaining or enhancing functional connectivity of these higher-order networks is crucial for meta-cognition (awareness of one’s thought process), as a limitation in ATP in one set of neurons can disrupt the synchronized neural firing within these disparate circuits and thus limit the individual’s ability to maintain focus and to generate new insights (Chen & Zhang, 2021). In this context, creatine supplementation may help buffer neuronal energy demands, thus supporting neural synchronization that underlies the advanced cognition necessary for deeper self-awareness and therapeutic insight. However, more studies are needed to adequately assess this hypothesis, as most studies in cognition assess cognitive battery tasks that do not test novel insight generation.
In addition to supporting neuroenergetics, creatine supplementation may exhibit antidepressant effects through several other mechanisms, including reducing glutamate-induced excitotoxicity and ROS species production, as well as potentiating synaptogenesis and synaptic plasticity.
Creatine Supplementation Enhances Cognitive Performance and Brain Function During Sleep Deprivation and Hypoxia
Emerging research demonstrates creatine supplementation significantly improves cognitive performance, executive functioning, and brain excitability under conditions of sleep deprivation and oxygen deprivation (hypoxia). This highlights creatine's potential as a neuroprotective supplement during periods of neural energy stress.
In a double-blind, randomized, placebo-controlled trial (McMorris et al., 2006), 19 healthy young adults received creatine supplementation (20 g/day, administered as four daily doses of 5 g) or placebo for 7 days, followed by 24 hours of sleep deprivation combined with mild intermittent exercise. Cognitive and psychomotor performance were assessed at baseline (0 h) and after 6, 12, and 24 hours of sleep deprivation using tests including random movement generation, verbal and spatial recall, choice reaction time, static balance, and mood state. At 24 hours, the creatine group showed significantly less impairment compared to placebo in performance on tasks reliant on executive function and psychomotor skills, including better random movement generation (Adjacency score significantly improved, p < 0.05), faster choice reaction times (~0 ms change vs. ~21 ms deterioration in placebo, p < 0.05), and improved static balance (fewer corrections needed, p < 0.01). Mood state was also better preserved with significantly lower reported fatigue (p < 0.005) and higher vigor (p < 0.02) in the creatine group. However, simpler short-term memory tasks (verbal and spatial recall) were unaffected by creatine supplementation. Plasma concentrations of norepinephrine and dopamine increased significantly after 24 hours of sleep deprivation, while cortisol levels significantly decreased, but these effects did not differ between the creatine and placebo groups. The study suggested creatine supplementation selectively benefits tasks that tax the prefrontal cortex under conditions of prolonged wakefulness and mild stress, likely due to enhanced energetic support in these brain regions.
In a subsequent double-blind, randomized, placebo-controlled trial, (McMorris et al. (2007) 19 healthy young adults were assigned to either creatine supplementation (20 g/day, provided as four daily doses of 5 g) or placebo for 7 days, followed by a 36-hour period of continuous wakefulness accompanied by intermittent moderate-intensity exercise. Creatine supplementation selectively improved performance only on the central executive random number generation task at the 36-hour time point, where the creatine group's performance significantly surpassed the placebo group’s (p < 0.05), with random number generation index (RNG) scores progressively improving from baseline to 36 hours in the creatine group (p < 0.01). In contrast, creatine supplementation did not significantly affect simpler cognitive tasks such as verbal recall or choice reaction time, nor did it affect dynamic balance, mood states, cognitive effort, or cortisol concentrations. These findings indicate creatine's cognitive benefits appear selective, occurring primarily under prolonged sleep deprivation conditions and specifically on complex executive tasks that heavily engage the prefrontal cortex.
In a randomized, double-blind, placebo-controlled crossover trial (Turner et al., 2015), 15 healthy adults supplemented with creatine (~20 g/day for 7 days) or placebo. Instead of sleep loss, subjects experienced acute hypoxia by breathing a gas mixture containing 10% oxygen for 90 minutes. Main outcomes included cognitive performance, specifically complex attentional capacity, corticomotor excitability assessed by transcranial magnetic stimulation (TMS), and subjective alertness or fatigue. Creatine supplementation significantly preserved cognitive performance during hypoxia, particularly complex attention tasks, and markedly enhanced corticomotor excitability by approximately 70% compared to placebo. Supplementation also effectively increased brain creatine concentrations by an average of 9.2%.
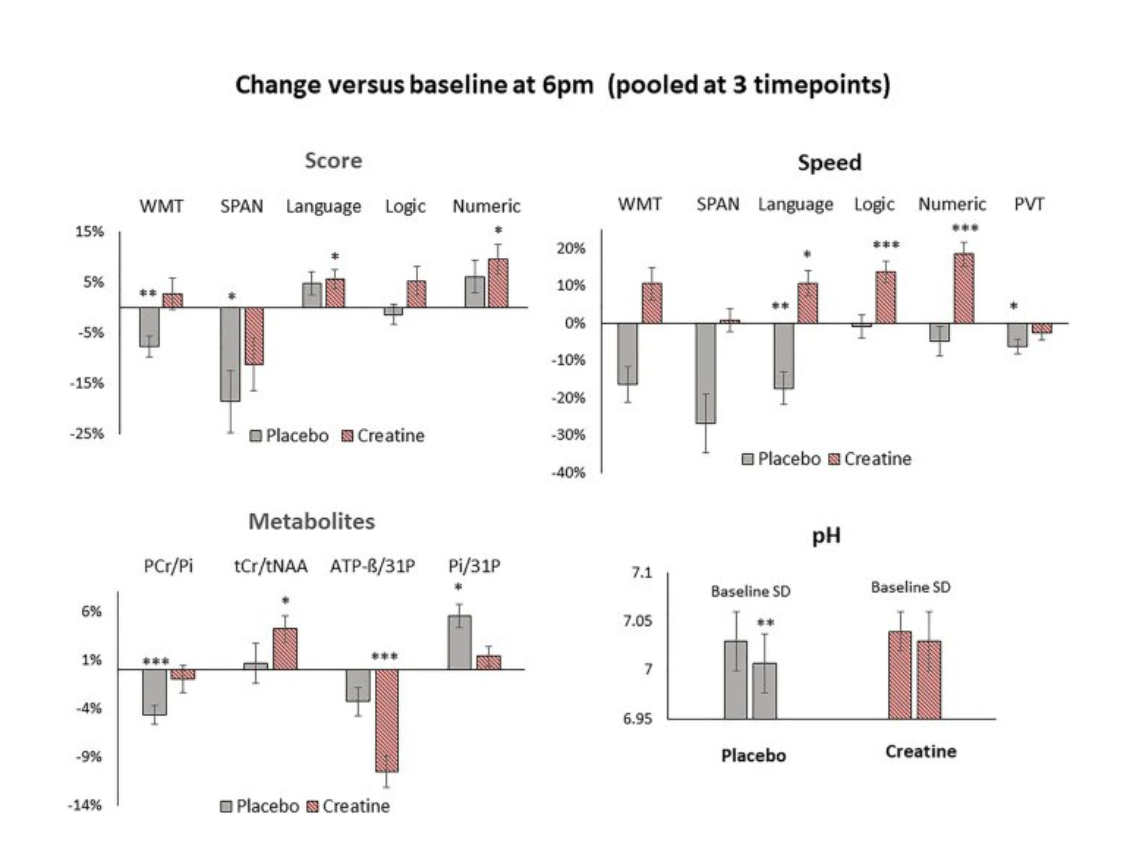
In a randomized, double-blind, placebo-controlled crossover trial (Gordji-Nejad et al., 2024), healthy adults received a single oral high-dose of creatine monohydrate (0.35 g/kg, ~25 g for a 70 kg individual) or placebo during 21 hours of sleep deprivation. Cognitive performance and cerebral energy metabolism were assessed at baseline and at 3.5, 5.5, and 7.5 hours post-supplementation using ^31P-MRS and ^1H-MRS imaging. Creatine supplementation significantly improved cognitive performance, enhancing processing speed by approximately 16–29% and memory by about 10%, while also reducing subjective fatigue by roughly 8%. At a metabolic level, creatine increased cerebral phosphocreatine (PCr) by ~4–6% and total creatine (tCr) by approximately 5%, reduced inorganic phosphate (Pi) by 8–10%, lowered ATP levels by up to 18%, and prevented the sleep deprivation-induced reduction in cerebral pH. These findings suggest a single high-dose creatine supplement effectively counteracts the cognitive and energetic impairments associated with acute sleep deprivation.
Creatine also improved cognitive performance and processing speed.
Note. Reprinted from “Single dose creatine improves cognitive performance and induces changes in cerebral high energy phosphates during sleep deprivation”, by Gordji-Nejad et al., 2024, Scientific Reports, 14, p. 4937.
Note. Reprinted from “Single dose creatine improves cognitive performance and induces changes in cerebral high energy phosphates during sleep deprivation”, by Gordji-Nejad et al., 2024, Scientific Reports, 14, p. 4937.
Taken together, these findings strongly support creatine's selective benefit for cognitive tasks demanding high executive functioning and attentional capacity under conditions of prolonged wakefulness and acute hypoxia. Creatine supplementation appears most beneficial when neural energy supply is compromised, suggesting potential clinical and practical applications for maintaining cognitive performance under stress.
Methods
Objective
This scoping review aims to map the existing literature on creatine supplementation in mental health across various psychiatric diagnostic categories, identifying key themes, research gaps, and implications for clinical practice and future research.
Framework
This review follows Arksey and O’Malley's methodological framework for scoping reviews, enhanced by the recommendations of the Joanna Briggs Institute (JBI) (Peters et al., 2015). It is reported in accordance with the PRISMA extension for Scoping Reviews (PRISMA-ScR) checklist (Tricco et al., 2018).
Identifying the Research Question
Our primary research question was: “What is known from the existing literature about the effects and implications of creatine supplementation across different psychiatric diagnostic categories?” In this study, we defined a scoping review as a research method designed to systematically explore the existing literature on a specific topic or field, with the goal of identifying key themes, research gaps, and various types of evidence that can guide practice, policy development, and future research (Munn et al., 2022).
Search Strategy
A comprehensive search was conducted in the following databases: PubMed, EMBASE, and Cochrane Library. Search terms included various combinations and synonyms of the following keywords:
Creatine or creatine supplementation AND mental health, psychiatric disorders, depression, anxiety, schizophrenia, bipolar disorder, cognitive disorders, neurodevelopmental disorders, PTSD, substance use disorders
The search was restricted to peer-reviewed articles published in English, with no limitations on date to ensure comprehensive literature coverage.
Study Selection
Studies were eligible if they met the following inclusion criteria:
Empirical studies (qualitative, quantitative, mixed methods)
Human studies across any age group
Studies specifically assessing creatine supplementation effects on mental health outcomes in any defined psychiatric diagnostic category.
Exclusion criteria included:
Animal studies
Non-original research (reviews, editorials, opinion pieces)
Studies with non-oral creatine administration routes
Non-English articles
Data Extraction
Data were systematically extracted using a standardized extraction template capturing:
Authors, publication year, and country
Study design and methodology
Diagnostic categories studied
Participant demographics (age, gender, clinical diagnosis, number)
Details of creatine supplementation (dosage, frequency, duration)
Outcome measures used
Summary of primary and secondary mental health outcomes
Reported adverse effects or safety concerns
Data Synthesis
Results were synthesized narratively to identify overarching themes, diagnostic-specific findings, methodological approaches, and gaps in the literature. The data were categorized and summarized according to psychiatric diagnostic groups to facilitate a clear understanding of the current evidence landscape.
Results
Reporting
This scoping review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist (Tricco et al., 2018), ensuring methodological transparency and completeness.
Study Selection Process
A total of 21,858 records were identified through database searches. After title and abstract screening of each record, 5.682 full-text articles were assessed for eligibility. Ultimately, 15 studies met inclusion criteria and were included in this review.
The study selection process is illustrated in Figure 1 (PRISMA-ScR flow diagram).
Figure 1. PRISMA-ScR flow diagram of study selection process.
Characteristics of Included Studies
The included studies spanned from 2006 to 2025, covering diverse psychiatric populations including depression, bipolar disorder, PTSD, schizophrenia, and substance use disorders. Most studies (n = 15) were randomized controlled trials, with additional open-label studies, pilot trials, and case reports.
Table 1 presents detailed characteristics of each study, including study design, sample size, diagnosis, creatine dosage, duration, and mental health outcomes assessed.
Table 1. Summary of Included Studies Evaluating Creatine Supplementation Across Psychiatric Disorders (n = 15)
Narrative Synthesis by Diagnostic Categories
Mood Disorders (Depression, Bipolar Disorder)
The majority of studies focused on depressive disorders, with creatine supplementation often evaluated as an adjunct to SSRIs, CBT, or other psychotherapies. Doses ranged from 3–10g/day, with durations of 4 to 8 weeks. Several studies reported statistically significant improvements in depression severity, though some findings were limited by small sample sizes and short durations (Sherpa et al., 2025; Lyoo et al., 2012).
Adjunctive Creatine Boosts CBT Effectiveness in Depression Treatment (Sherpa et al., 2025)
In this double-blind, randomized, placebo-controlled feasibility trial, researchers investigated the effects of oral creatine monohydrate supplementation as an adjunct to cognitive behavioral therapy (CBT). 100 adults with major depressive disorder (MDD) received biweekly CBT with either 5g/day of creatine or placebo for 8 weeks. The creatine group saw a greater reduction in PHQ-9 scores, with an adjusted mean difference of −5.12 (95% CI: −7.20 to −3.52; p < 0.05). Practically, this means that, by adding creatine to CBT, several symptoms like “little interest or pleasure in doing things” would go from “more than half the days” to “several days” in a two week period. Of note, the creatine group was also found to have lower treatment discontinuation rates and no significant increase in adverse events when compared to placebo. However, diarrhea (15 vs. 6 reports) and abdominal pain (9 vs. 1 reports) were more frequently reported in the creatine group, while vomiting (2 vs. 9 reports) and pruritus (4 vs. 11 reports) were more common in the placebo group. These findings support creatine’s safety and tolerability. This study was done on a mixed-gender population (50% female; mean age ~30 years old), and is one of the few on creatine supplementation in a lower-resourced clinical setting. Below are some of the data from this study as mentioned above.
Note. Reprinted from “Efficacy and safety profile of oral creatine monohydrate in add-on to cognitive-behavioural therapy in depression: An 8-week pilot, double-blind, randomised, placebo-controlled feasibility and exploratory trial in an under-resourced area”, by Sherpa et al., 2025, European Neuropsychopharmacology, 90, p. 32.
Creatine Augmentation Enhances Antidepressant Efficacy in Women with Depression (Lyoo et al., 2012)
This 8-week double-blind, placebo-controlled RCT recruited 52 women with MDD. Participants received escitalopram in addition to either creatine or placebo. The dosage of creatine started at 3g/day for week 1, going up to 5g/day from weeks 2-8. Four weeks into the study, mean Hamilton Depression Rating Scale (HAM-D) scores dropped 21.8% more in the creatine group versus the placebo group. By week 8, mean HAM-D scores had decreased from 26.9 to 5.4 in the creatine group, versus 26.7 to 9.8 in the placebo group (p < 0.001) Researchers reported an effect size of Cohen’s d = 1.13 for the difference in HAM-D score reduction from baseline to the end of the study (8 weeks). By week 8, significantly more participants in the creatine group achieved remission compared to placebo (52.0% vs. 25.9%; p = 0.008).
Note. Reprinted from “A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder”, by Lyoo et al., 2012, The American journal of psychiatry, 169(9), p. 941.
Creatine Shows Higher Remission Rates for Bipolar Depression Despite Mixed Results on Symptom Reduction (Toniolo et al., 2018)
In this randomized, double-blind, placebo-controlled trial, Toniolo et al. (2018) investigated the efficacy of creatine monohydrate (6g/day) as an adjunctive treatment for patients with bipolar disorder type I or II experiencing a depressive episode. Over the course of 6 weeks, patients either received 6g/day of creatine or placebo. Efficacy was primarily measured using the Montgomery–Åsberg Depression Rating Scale (MADRS) scores. While a statistically significant difference in score reduction was not found between the groups (p = 0.560; Cohen’s d = 0.231), the creatine group did see a significantly greater rate of remission (defined as MADRS score ≤ 12). On ITT analysis, the creatine group saw a remission rate of 52.9% versus the placebo group’s rate of 11.1% (p = 0.012, OR = 9.0). Of note, one patient from the creatine group experienced a hypomanic switch, and another had a manic switch.
Note. Reprinted from “A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression”, by Toniolo et al., 2018, Journal of Neural Transmission, 125, p. 253.
Creatine Improves Depression Symptoms and Boosts Brain Energy Metabolism in SSRI-Resistant Adolescents (Kondo et al., 2011)
In this open-label pilot study by Kondo et al. (2011), five female adolescents (ages 14-18) with SSRI-resistant major depressive disorder (MDD) received adjunctive treatment of creatine monohydrate at 4g/day for 8 weeks. All participants continued their stable fluoxetine treatment throughout the study. Depression severity was measured using the Children’s Depression Rating Scale–Revised (CDRS-R), which saw mean scores drop from 69.0 at baseline to 30.6 by the end of the study (56% decline). The study also utilized 31-phosphorus magnetic resonance spectroscopy (31P-MRS) to measure brain phosphocreatine levels, which showed a significant increase of 6.4% in the frontal lobe of participants when compared to scans of healthy female adolescents (p = 0.02). This study was among the first to report measurable increases in brain PCr associated with creatine supplementation in a psychiatric population. However, its small sample size and lack of a placebo control limit broader generalizability.
Creatine Supplementation Shows Rapid Antidepressant Effects but Potential Risk of Manic Switch in Bipolar Disorder (Roitman et al., 2007)
This was a 4-week open-label trial of oral creatine monohydrate (titrated from 3g to 5g/day) supplementation in 8 patients with unipolar depression and 2 with bipolar depression. Of the ten participants, one significantly improved after the first week and withdrew, and the two patients with bipolar disorder experienced manic/hypomanic switches, also withdrawing early; therefore, statistical analyses included only the seven remaining unipolar participants who completed at least three weeks. Participants showed significant improvement across multiple scales, with mean HAM-D dropping from 23.14 to 12.57 (p = 0.002), mean Clinical Global Impression (CGI) scores dropping from 4.43 to 3.00 (p = 0.02), and mean Hamilton Anxiety Scale (HAS) scores dropping from 18.71 to 12.00 (p = 0.016).
Creatine Reduces Depression and Methamphetamine Use While Increasing Brain Energy in Females with Dual Diagnosis (Hellem et al., 2015)
In this open-label pilot study, 14 women with Major Depressive Disorder and active methamphetamine use were given 5g/day creatine monohydrate over the course of 8 weeks. Eleven participants (78.6%) completed the trial, experiencing significant reductions in mean HAM-D scores from 16.86 at baseline to 7.36 by week 8 (56.4% decrease). Participants also underwent 31-Phosphorus-magnetic resonance spectroscopy, which showed an increase in mean frontal lobe PCr from 0.223 to 0.233 (p < 0.01, Cohen’s d = 0.92). Additionally, significant reductions in anxiety (Beck Anxiety Inventory scores) were seen as early as week 2 and sustained through study completion. Methamphetamine use also decreased by over 50% by week 6, as measured by urine drug screens. This was the first study to show the potential effectiveness of creatine in a dual-diagnosis population.
Creatine Monohydrate Increases Brain Phosphocreatine and Correlates with Improved Depression Symptoms in SSRI-Resistant Adolescent Females (Kondo et al., 2016)
This randomized, placebo-controlled, dose-ranging study recruited 34 adolescent females aged 13-20 years with SSRI-resistant depression, assigning each to 2g, 4g, or 10g/day of either creatine or placebo for 8 weeks. In contrast to the studies discussed above, depression scores (CDRS-R scores, in this study) were the secondary outcome in this study. The primary outcome was frontal lobe phosphocreatine as measured on 31P-MRS. The mean frontal lobe PCr increased by 4.6%, 4.1%, and 9.1% in the 2g, 4g, and 10g groups respectively, while the placebo group showed a decrease of 0.7%. However, these differences did not reach statistical significance between groups (p = 0.69), and the study may have been underpowered to detect statistically significant differences. While researchers did not find a statistically significant between-group difference in CDRS-R score reduction, regression analysis did reveal an inverse correlation between frontal lobe PCr and depression scores (p = 0.02). Creatine supplementation was generally well-tolerated, with no significant differences in adverse effects, weight gain, or renal function between groups.
Note. Reprinted from “Creatine target engagement with brain bioenergetics: a dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression”, by Kondo et al., 2016, Amino Acids, 48, p. 1948.
Creatine Augmentation Shows No Significant Benefit for SSRI-Resistant Depression but May Induce Rapid Improvement in Select Female Patients (Nemets & Levine, 2013)
This 4-week, randomized, double-blind, placebo-controlled, dose-finding trial investigated oral creatine as an add-on to ongoing antidepressant treatment in 18 adults with MDD refractory to SSRIs, SNRIs, or NASA antidepressants (e.g. citalopram, venlafaxine, mirtazapine). Participants were divided into 4 groups, receiving either creatine or placebo of up to 5g/day or 10g/day. Mean HAM-D scores were measured, which saw a decrease in all who received creatine from 27.6 to 13.4 (versus 28.2 to 15.3 in the placebo group); however, these findings were not statistically significant (p = 0.4). Researchers also find no dose effect.
Note. Reprinted from “A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression”, by Nemets & Levine, 2013, International clinical psychopharmacology, 28(3), pp. 127-133.
Creatine Supplementation Enhances Brain Metabolism, Rich-Club Networks, and Depression Outcomes in Women with MDD (Yoon et al., 2016)
This study was an extension of the Lyoo et al. (2012) RCT discussed above. Researchers studied the effects of 5g/day of oral creatine in women with MDD who were undergoing treatment with escitalopram. Of the 52 participants, 34 agreed to additionally undergo pre- and post-treatment neuroimaging via ¹H-MRS and diffusion tensor imaging (DTI). Metabolic and network outcomes were measured for changes in prefrontal N-acetylaspartate, a marker of neuronal viability and mitochondrial function. Participants in the creatine group saw significant increase in NAA versus the placebo group (p = 0.01, Cohen’s d = 0.73) and versus healthy controls (p = 0.03, Cohen’s d = 0.66). “Rich-club” networks (sets of highly connected brain regions associated with efficient global communication, reported to expend large amounts of metabolic energy, thus rendering them vulnerable to altered bioenergetics) were also measured, showing a significant increase in the creatine group versus placebo group and healthy controls (p = 0.01, Cohen’s d = 0.79; p = 0.03, Cohen’s d = 0.64). All of these findings on neuroimaging correlated with greater improvements in depressive symptoms.
Note. Reprinted from “ Effects of creatine monohydrate augmentation on brain metabolic and network outcome measures in women with major depressive disorder.”, by Yoon et al., 2016, Biological Psychiatry, 80(6), pp. 439–447.
Note. Reprinted from “ Effects of creatine monohydrate augmentation on brain metabolic and network outcome measures in women with major depressive disorder.”, by Yoon et al., 2016, Biological Psychiatry, 80(6), pp. 439–447.
Creatine and 5-HTP Combination Significantly Improves Depression in SSRI/SNRI-Resistant MDD (Kious et al., 2017)
This was an open-label, 8-week pilot study investigating the effects of creatine monohydrate in conjunction with 5-hydroxytryptophan as adjunctive treatment in 15 women with SSRI/SNRI-resistant MDD. Participants received 5/g day of oral creatine monohydrate and 100 mg twice daily of 5-HTP. Depression severity was measured using HAM-D, showing significant improvement with mean scores dropping from 18.9 at baseline to 7.5 by the conclusion of the study (p < 0.00001).
Note. Reprinted from “An open-label pilot study of combined augmentation with creatine monohydrate and 5-hydroxytryptophan for selective serotonin reuptake inhibitor–or serotonin-norepinephrine reuptake inhibitor–resistant depression in adult women”, by Kious et al., 2017, Journal of clinical psychopharmacology, 37(5), pp.578-583.
Creatine Improves Verbal Fluency but Not Mood Symptoms in Bipolar Depression (Toniolo et al., 2017)
This 6-week, randomized, double-blind, placebo-controlled trial investigated the effects of 6g/day of creatine monohydrate on mood symptoms and cognitive performance in 18 participants (ages 18-59) with bipolar depression. Patients received either creatine or placebo across the 6 weeks, in addition to their prior established mood-stabilizing regimens. MADRS and HAM-D scores were measured at baseline and at the end of the study, with no statistically significant differences between treatment groups (p = 0.496). However, there was a significant improvement in the FAS verbal fluency test for the creatine group (p = 0.017, Cohen’s d = 1.252). Other neuropsychological tests were also administered, including the Wisconsin Card Sorting Test (assesses cognitive flexibility and problem-solving) and the Stroop test (measures attention and inhibitory control), both of which did not show statistically significant differences between groups.
Creatine Improves Mood, Pain, and Quality of Life in Treatment-Resistant Depression (Amital et al., 2006b)
This case report studied the effects of oral creatine monohydrate supplementation in a 52-year-old woman with treatment-resistant depression, PTSD, and fibromyalgia. The patient received 3g/day of creatine in week 1, increasing to 5g/day for weeks 2 through 4. Additionally, she continued to receive her ongoing pharmacotherapy with citalopram. Throughout the study, her HAM-D score dropped from 24 to 16 (33% decrease). Her pain severity was also measured using the Visual Analog Scale (VAS), which saw a decline from 80 to 40. The Short Form 36 Health Status Questionnaire (SF-36) was used to measure her quality of life, and her score increased by 30% throughout the study. Of note, the patient noted improved sleep and cognitive functioning, and requested to continue creatine supplementation after the conclusion of the study.
Creatine Supplementation Shows Modest Benefits for Anxiety Symptoms as Secondary Outcome
Few studies directly assessed anxiety outcomes. Where measured, anxiety symptoms tended to improve in parallel with depression, but no studies focused on anxiety as a primary endpoint. For example, in studies primarily focused on depression or PTSD, anxiety symptoms were assessed as secondary outcomes using instruments like the Hamilton Anxiety Rating Scale (HAM-A) or Beck Anxiety Inventory (BAI), with modest improvements reported (Amital et al., 2006a; Kious et al., 2017).
Creatine Supplementation Yields Inconsistent Results for Schizophrenia Treatment
Two studies evaluated creatine supplementation in individuals with schizophrenia. One RCT (Kaptsan et al., 2007) found no significant benefits, while an open-label study (Levental et al., 2015) showed potential improvement in schizophrenia symptoms with high-dose creatine. After 6 months of daily creatine supplementation, PANSS (Positive and Negative Syndrome Scale) scores and PANSS general subscale scores showed significant reduction from 92.6 to 87.4 (-5.2) and 46.9 to 43.9 (-3.0), respectively (p = 0.004 and p = 0.021, respectively).
Creatine Supplementation Does Not Improve Schizophrenia Symptoms in Double-Blind, Placebo-Controlled Trial (Kaptsan et al., 2007)
This was a randomized, double-blind, placebo-controlled, crossover study on the effects of oral creatine monohydrate on 12 patients diagnosed with schizophrenia. Patients received either creatine (3g/day for the first month, 5g/day for the following two months) or placebo for 3 months at a time, after which they would switch to the alternative treatment for another 3 months. There were several outcomes measured, including the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impressions (CGI) scale, and Abnormal Involuntary Movement Scale (AIMS). There were no statistically significant differences found between creatine and placebo for any of these scales. Creatine supplementation was well tolerated, with the main side effect noted being nausea in two of the patients.
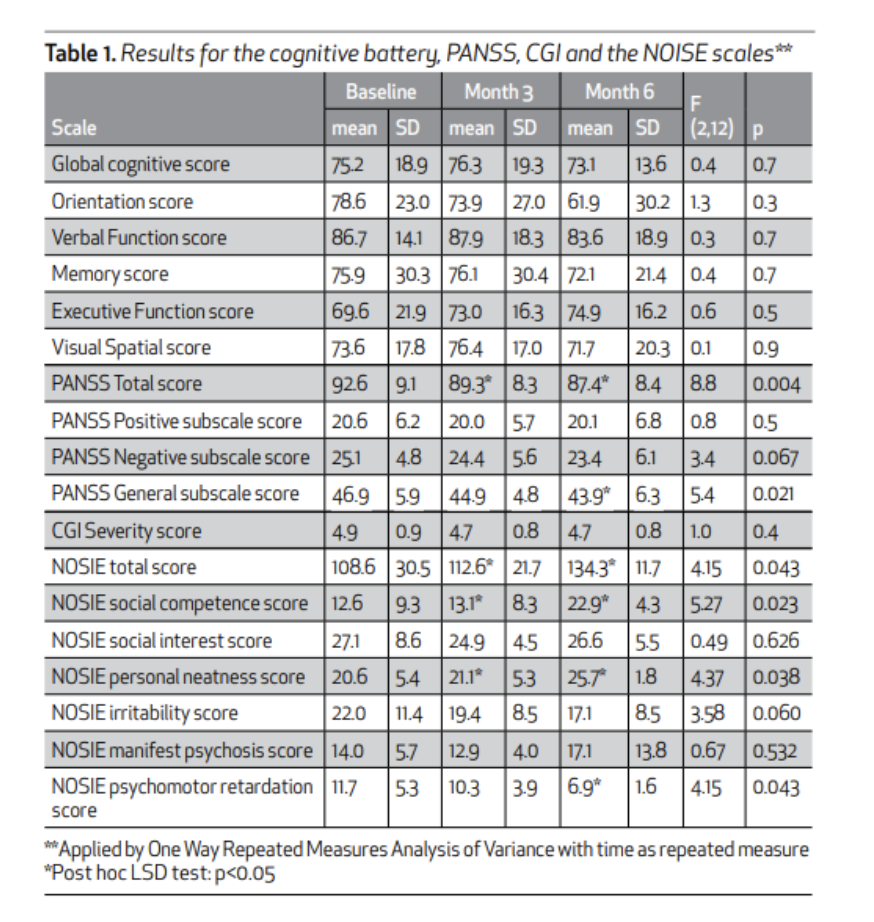
High-Dose Creatine Improves Negative Symptoms and Ward Behavior in Treatment-Resistant Schizophrenia (Levental et al., 2015)
This was a 6-month, open-label, pilot study investigating high-dose creatine monohydrate augmentation in 7 male patients (age 35-55) with schizophrenia and treatment-resistant negative symptoms. Creatine dosing was titrated starting at 3g/day (weeks 1–2), 5g/day (weeks 3–4), 7g/day (weeks 5–6), and finally 10g/day (week 7 onward). Several outcomes were measured across the study, including the Positive and Negative Symptoms Scale (PANSS), Clinical Global Impressions (CGI), Nurse Observation Scale for Inpatient Evaluation (NOSIE; a sensitive rating scale for ward behavior), Extrapyramidal Symptom Rating Scale (ESRS, designed to rate the severity of four types of drug-induced movement disorders), and the Mindstreams computerized cognitive battery (a validated computerized neuropsychological test battery assessing memory, attention, executive function, and processing speed). Researchers found a significant change in mean total PANSS scores with creatine augmentation (p = 0.004); however, it was noted that this may have limited clinical value due to there only being mild improvement in the mean general psychopathology PANSS subscale scores (46.9 at baseline to 43.9 by the end of month 6, p = 0.021). Mean total NOSIE scores also saw a significant improvement (108.6 to 134.3, p = 0.043), particularly in personal neatness (p = 0.038), psychomotor retardation (p = 0.043), and social competence (p = 0.023).
Note. Reprinted from “ A pilot open study of long term high dose creatine augmentation in patients with treatment resistant negative symptoms schizophrenia”, by Levental et al., 2015, Israel Journal of Psychiatry, 52(1). p. 9.
Creatine Improves PTSD Symptoms and Quality of Life in Small Clinical Studies
Two studies examined PTSD: an open-label trial and a case study (Amital et al., 2006a & 2006b), both of which reported symptom improvement when creatine was added to ongoing treatment. In the open-label trial (Amital et al., 2006a), total CAPS (Clinician Administered PTSD Scale) scores saw an average 5.8 point reduction (p = 0.003). The case study was focused on a patient with comorbid PTSD, depression, and fibromyalgia (Amital et al., 2006b). While there was no formal PTSD symptom scale used in the study, there was a 30% improvement in overall quality of life as measured by the SF-36 Health Status Questionnaire, particularly in domains related to vitality, social functioning, and mental health.
Creatine Supplementation Reduces PTSD and Depressive Symptoms in Open-Label Trial (Amital et al., 2006a)
This was a 4 week, open-label pilot study investigating the effects of oral creatine monohydrate in 10 patients (8 men, 2 women; ages 43-61) diagnosed with PTSD. Creatine dosing was 3g/day for week 1 and 5g/day for weeks 2 through 4 (in addition to ongoing psychiatric treatment). The Clinician Administered PTSD Scale (CAPS) was used to assess PTSD severity, and total scores dropped from 75.6 at baseline to 69.8 at the end of week 4 (p = 0.003). Depressive symptoms were also measured using the HAM-D scale, with scores also dropping from 24.1 to 20.4 (p = 0.006).
Creatine Supplementation Reduces Depression and Increases Brain Energy but Not Methamphetamine Use in Substance Use Disorder
One pilot study discussed above (Hellem et al., 2015) evaluated creatine as an adjunctive treatment in females with methamphetamine dependence and comorbid depression. While methamphetamine use was monitored via urine drug screen and self-reports, there were no significant reductions in use throughout the study. Researchers did find a significant reduction in depressive symptoms, with mean HAM-D scores dropping from 16.9 to 7.36. Brain phosphocreatine levels were also measured during the study via phosphorus magnetic resonance spectroscopy, with levels significantly increasing from 0.223 to 0.233 (p < 0.01). This suggests increased brain energy metabolism associated with creatine supplementation.
Cognitive and Neurodevelopmental Disorders (e.g., Autism, ADHD)
No RCTs were found in ADHD or learning disorders.
Summary Of Outcomes And Themes
Among all psychiatric diagnoses reviewed, creatine supplementation demonstrated the greatest benefit in reducing depressive symptoms, particularly when used as an adjunctive treatment alongside established antidepressant medications or psychotherapies. Cognitive outcomes were less consistently measured but showed significant improvements in specific cognitive domains, notably verbal fluency, processing speed, and attentional capacity in selected studies. Across the reviewed literature, study designs, sample sizes, and creatine dosages varied considerably (ranging from 3g/day to 10g/day). Given previous studies reviewed on sleep deprivation, hypoxia, and animal models, it appears that many studies included in this review may have utilized suboptimal dosing strategies. Higher creatine doses—approximately 20 grams/day used in human studies involving sleep and oxygen deprivation, and doses proportionally higher in animal models—suggest potential underdosing in several psychiatric studies, which could partly explain mixed findings and modest effect sizes.
Common methodological limitations throughout included small sample sizes, short trial durations, open-label designs, absence of placebo controls in some studies, and limited diversity in patient populations. Adverse effects were generally minimal and infrequent; however, two studies involving bipolar patients (Roitman et al., 2007; Toniolo et al., 2018) reported cases of manic or hypomanic switches, highlighting the need for cautious monitoring in bipolar populations.
The bulk of available evidence focuses on depressive disorders, resulting in substantial research gaps in other psychiatric conditions, including anxiety disorders, PTSD, schizophrenia, substance use disorders, and neurodevelopmental disorders such as ADHD and autism. Future studies should aim to address these gaps through rigorous randomized controlled trials with adequately powered sample sizes, appropriate placebo controls, diverse patient populations, longer treatment durations, and consideration of higher-dose creatine supplementation strategies supported by prior literature.
Discussion
Overview of Included Studies
This scoping review analyzed 15 different studies across 5 diagnostic categories, with mood disorders seeing the greatest representation (10 articles primarily studying MDD, 2 articles focused on bipolar disorder). Several of the studies investigated patient populations with multiple diagnoses (e.g. MDD and SUD). Of note, there were no studies found focusing on creatine supplementation in anxiety disorders. The majority of the studies utilized creatine as an adjunct therapy alongside ongoing psychiatric treatments (e.g. CBT, SSRIs, etc.). Creatine dosage ranged from 3-10g/day, with most studies titrating up from a lower initial dose. Across the mood disorder studies, creatine consistently improved depressive symptoms, with multiple studies reporting statistically significant reductions in mean PHQ-9, HAM-D, and MADRS scores. Neuroimaging outcomes were also measured in 3 studies (via 31P-MRS or 1H-MRS), which saw increased PCr and NAA levels when measuring after creatine supplementation.
Interpretation and Clinical Implications
Creatine’s potential role in psychiatry is supported by biological rationale grounded in its function as a cellular energy buffer. It facilitates rapid regeneration of ATP via the PCr system. Regions of the brain that have high metabolic activity, such as the prefrontal cortex and hippocampus, rely on this mechanism in order to regulate emotion and cognition. Studies have shown creatine also having the ability to enhance gamma-band oscillations, support astrocyte-neuron energy transfer, and stabilize mitochondrial function—all of which contribute to maintaining neural efficiency and resilience under stress (Chen & Zhang, 2021). This aligns with the bioenergetic framework proposed by Fabiano and Stubbs (2025), who argue that depression involves mitochondrial dysfunction and ATP instability under chronic stress, and that creatine may restore energetic balance and promote neuroplasticity.
Clinically, creatine has shown promise as an adjunctive treatment across various psychiatric populations, particularly in unipolar and bipolar depression, where it has been associated with reductions in symptom severity and improvements in cognitive domains such as verbal fluency. Given prior evidence from human studies involving sleep and oxygen deprivation (using doses around 20 g/day), as well as animal studies employing significantly higher doses, many of the reviewed psychiatric studies may have used suboptimal creatine dosing, potentially underestimating its therapeutic efficacy. Its strong safety profile—supported by consistent tolerability across studies with doses ranging from 3g to 10g/day—makes it an appealing low-risk intervention, especially in low-resource or integrative care settings. While no serious adverse events were reported in the studies reviewed, isolated cases of manic switching in bipolar participants underscore the need for monitoring and further investigation in this population. Overall, the convergence of clinical, cognitive, and neurobiological data suggests that creatine holds meaningful therapeutic potential and warrants further study as a complementary psychiatric intervention.
Strengths and Limitations of This Scoping Review
This scoping review adhered to pre-specified protocol as specified by the Arksey and O’Malley framework and PRISMA-ScR checklist. Our search strategy applied comprehensive coverage by searching for studies from five different databases (PubMED, EMBASE, Cochrane Library, PsycINFO, and Web of Science), with manual reference list screening, citation tracking and keyword refinement across multiple diagnostic categories. Our inclusion criteria were clearly defined, with relevance screening occurring subsequently. Data extraction methods were standardized, focusing on detailed information on study design, dosing, outcomes, and effect sizes. Additional synthesis was done by diagnostic group and outcome domain (e.g. neuroimaging, cognition). This review is among the first to systematically explore studies investigating creatine as a supplement to psychiatric disorders. To our knowledge, this is one of the first reviews to systematically examine the use of creatine supplementation across psychiatric disorders, and the most comprehensive.
Despite our broad inclusion criteria, our review focused only on oral creatine monohydrate supplementation in human studies. Excluding animal research/studies, or IV/topical creatine administration may have caused us to miss out on potentially informative outcomes. The variety of the studies reviewed (sample size, outcome measures, duration, etc.) limited our ability to synthesize findings quantitatively or conduct formal comparisons between studies. Most of the studies had small sample sizes and were open-label designs, both of which increase risk of bias and decrease generalizability. Limiting our search to English-language studies may have prevented us from incorporating relevant research published in other languages or in gray literature. While screening and data were conducted systematically, interpretation of findings was partially subject to reviewer judgment.
Recommendations
Future research on creatine supplementation in psychiatric populations should implement larger, multicenter randomized controlled trials to robustly confirm efficacy across diverse diagnostic groups. Future studies should explicitly investigate anxiety disorders and psychotic disorders, given the notable scarcity of research in these areas. Optimal creatine dosing (including higher doses such as 10g to 20g/day) and longer treatment durations (e.g., 10–12 weeks or longer) should be systematically explored to identify the most effective supplementation protocols. Objective biomarkers (e.g., 31P-MRS, cognitive batteries) should continue to be incorporated to elucidate the biological mechanisms underlying treatment response. Long-term safety data would be invaluable, especially for patient subgroups at higher risk of adverse outcomes (e.g., manic switching in bipolar disorder).
While creatine’s mood-related effects have been more widely studied, its potential to enhance cognitive performance under stress remains an important area for future investigation. Preliminary studies suggest that creatine may selectively improve executive function in energy-demanding conditions such as sleep deprivation and oxygen deprivation (McMorris et al., 2007; Turner et al., 2015). These effects appear to be task-dependent, with benefits most pronounced for prefrontal-mediated functions like attention and working memory, rather than simpler recall tasks (McMorris et al., 2007). This specificity points to the value of studying creatine in psychiatric and neurologic populations that experience cognitive slowing, attentional deficits, or neuroenergetic dysfunction. Future trials should incorporate standardized cognitive testing and, where feasible, neuroimaging modalities such as ³¹P-MRS to evaluate whether cognitive benefits correspond with changes in brain energy metabolism (Gordji-Nejad et al., 2024).
Conclusion
This comprehensive scoping review highlights creatine monohydrate as a promising adjunctive therapeutic intervention for various psychiatric conditions, particularly demonstrating robust efficacy in mood disorders such as unipolar and bipolar depression. Creatine supplementation consistently improved depressive symptoms, enhanced cognitive function, and supported critical neuroenergetic processes, including elevated brain phosphocreatine and neuronal viability. Notably, studies employing rigorous methodologies, such as randomized controlled trials with neuroimaging biomarkers, reinforce the validity of these findings.
Despite its demonstrated potential, current studies often employ lower dosages (3–10g/day) than those proven effective in neurophysiologically demanding conditions such as sleep deprivation and hypoxia, or in animal models where higher dosages yielded greater therapeutic benefits. Human neuroimaging studies have shown that creatine doses between 2–5g/day typically produce modest brain phosphocreatine increases of approximately 4–6%, while higher doses of around 10g/day for 8 weeks or 20g/day for 7 days have resulted in more substantial elevations (approximately 9–10%) in brain creatine concentrations, correlating with enhanced cognitive function and reduced fatigue. Therefore, future research should rigorously investigate higher-dose regimens and extended supplementation durations to optimize therapeutic outcomes. Additionally, expanding the scope to address under-examined psychiatric disorders—particularly anxiety disorders and psychosis—could uncover broader clinical applications for creatine.
Creatine’s strong safety and tolerability profile, combined with its accessibility and low cost, position it uniquely as an attractive adjunctive treatment, especially relevant in resource-limited settings. Nevertheless, vigilance regarding potential adverse effects, such as manic switching in vulnerable bipolar populations, remains crucial.
Importantly, creatine supplementation represents just one piece of a broader strategy aimed at improving metabolic function. Future research should prioritize exploring combinations of interventions, such as integrating creatine with structured exercise programs, sauna, dietary interventions including ketogenic diets, and other metabolic-enhancing therapies. Given the substantial evidence supporting exercise's role in enhancing cognitive health, promoting neurogenesis, and reducing inflammation, (Episode 165) future studies should particularly emphasize combining creatine supplementation with structured exercise programs to optimize cognitive and psychiatric outcomes. Such integrative approaches could potentially yield more comprehensive improvements in psychiatric and cognitive outcomes.
In summary, the existing evidence compellingly supports creatine monohydrate’s role as a viable adjunctive treatment capable of significantly enhancing mood, cognitive performance, and neurobiological resilience across psychiatric populations. To fully capitalize on creatine's therapeutic potential, forthcoming research must prioritize robust, adequately powered clinical trials exploring optimized dosing strategies, long-term outcomes, integrative metabolic therapies, and broader psychiatric applications. Consequently, clinicians and researchers alike should consider creatine supplementation not merely as an experimental option, but as a scientifically substantiated intervention poised to substantially augment existing psychiatric treatment paradigms.
References:
Adhihetty, P. J., & Beal, M. F. (2008). Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular medicine, 10(4), 275–290. https://doi.org/10.1007/s12017-008-8053-y
Ahn, N., Leem, Y. H., Kato, M., & Chang, H. (2016). Effects of creatine monohydrate supplementation and exercise on depression-like behaviors and raphe 5-HT neurons in mice. Journal of Exercise Nutrition & Biochemistry, 20(3), 24–31. https://doi.org/10.20463/jenb.2016.09.20.3.4
Amital, D., Vishne, T., Roitman, S., Kotler, M., & Levine, J. (2006a). Open Study of Creatine Monohydrate in Treatment-Resistant Posttraumatic Stress Disorder. The Journal of Clinical Psychiatry, 67(5), 836–837. https://doi.org/10.4088/jcp.v67n0521c
Amital, D., Vishne, T., Rubinow, A., & Levine, J. (2006b). Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. American Journal of Psychiatry, 163(10), 1840-1841. https://doi.org/10.1176/ajp.2006.163.10.1840b
Arksey, H., & O'Malley, L. (2005). Scoping Studies: Towards a Methodological Framework. International Journal of Social Research Methodology: Theory & Practice, 8(1), 19–32. https://doi.org/10.1080/1364557032000119616
Attwell, D., & Laughlin, S. B. (2001). An energy budget for signaling in the grey matter of the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 21(10), 1133–1145. https://doi.org/10.1097/00004647-200110000-00001
Avgerinos, K. I., Spyrou, N., Bougioukas, K. I., & Kapogiannis, D. (2018). Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Experimental gerontology, 108, 166-173. https://doi.org/10.1016/j.exger.2018.04.013
Chen, Y., & Zhang, J. (2021). How energy supports our brain to yield consciousness: Insights from neuroimaging based on the neuroenergetics hypothesis. Frontiers in Systems Neuroscience, 15, 648860. https://doi.org/10.3389/fnsys.2021.648860
Dedeoglu, A., Kubilus, J. K., Yang, L., Ferrante, K. L., Hersch, S. M., Beal, M. F., & Ferrante, A. R. J. (2003). Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. Journal of Neurochemistry, 85(6), 1359–1367. https://doi.org/10.1046/j.1471-4159.2003.01706.x
Fabiano, N., & Stubbs, B. (2025). Creatine as a treatment for depression: A brain bioenergetics perspective. European Neuropsychopharmacology, 96, 3-4. https://doi.org/10.1016/j.euroneuro.2025.03.014
Faulkner, P., Paioni, S. L., Kozhuharova, P., Orlov, N., Lythgoe, D. J., Daniju, Y., Morgenroth, E., Barker, H., & Allen, P. (2021). Relationship between depression, prefrontal creatine and grey matter volume. Journal of Psychopharmacology, 35(12), 1464–1472. https://doi.org/10.1177/02698811211050550
Forbes, S. C., Cordingley, D. M., Cornish, S. M., Gualano, B., Roschel, H., Ostojic, S. M., Rawson, E. S., Roy, B. D., Prokopidis, K., Giannos, P., & Candow, D. G. (2022). Effects of Creatine Supplementation on Brain Function and Health. Nutrients, 14(5), 921. https://doi.org/10.3390/nu14050921
Gordji-Nejad, A., Matusch, A., Kleedörfer, S., Patel, H. J., Drzezga, A., Elmenhorst, D., Binkofski, F., & Bauer, A. (2024). Single dose creatine improves cognitive performance and induces changes in cerebral high energy phosphates during sleep deprivation. Scientific Reports, 14, 4937. https://doi.org/10.1038/s41598-024-54249-9
Hellem, T. L., Sung, Y.-H., Shi, X.-F., Pett, M. A., Latendresse, G., Morgan, J., Huber, R. S., Kuykendall, D., Lundberg, K. J., & Renshaw, P. F. (2015). A pilot study of creatine as a novel treatment for depression in methamphetamine using females. Journal of Dual Diagnosis, 11(3–4), 189–195. https://doi.org/10.1080/15504263.2015.1100471
Hoskins, M., Pearce, J., Bethell, A., Dankova, L., Barbui, C., Tol, W. A., van Ommeren, M., de Jong, J., Seedat, S., Chen, H., & Bisson, J. I. (2015). Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. The British journal of psychiatry : the journal of mental science, 206(2), 93–100. https://doi.org/10.1192/bjp.bp.114.148551
Howes, O. D., Thase, M. E., & Pillinger, T. (2022). Treatment resistance in psychiatry: state of the art and new directions. Molecular Psychiatry, 27(1), 58-72. https://doi.org/10.1038/s41380-021-01200-3
Joo, P., Lee, H., Wang, S., Kim, S., & Hudetz, A. G. (2021). Network Model With Reduced Metabolic Rate Predicts Spatial Synchrony of Neuronal Activity. Frontiers in computational neuroscience, 15, 738362. https://doi.org/10.3389/fncom.2021.738362
Juneja, K., Bhuchakra, H. P., Sadhukhan, S., Mehta, I., Niharika, A., Thareja, S., Nimmakayala, T., & Sahu, S. (2024). Creatine Supplementation in Depression: A Review of Mechanisms, Efficacy, Clinical Outcomes, and Future Directions. Cureus, 16(10), e71638. https://doi.org/10.7759/cureus.71638
Kaptsan, A., Odessky, A., Osher, Y., & Levine, J. (2007). Lack of efficacy of 5 grams daily of creatine in schizophrenia: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry, 68(6), 881-884. https://doi.org/10.4088/jcp.v68n0609
Kious, B. M., Sabic, H., Sung, Y. H., Kondo, D. G., & Renshaw, P. (2017). An open-label pilot study of combined augmentation with creatine monohydrate and 5-hydroxytryptophan for selective serotonin reuptake inhibitor–or serotonin-norepinephrine reuptake inhibitor–resistant depression in adult women. Journal of clinical psychopharmacology, 37(5), 578-583. https://doi.org/10.1097/JCP.0000000000000754
Kondo, D. G., Sung, Y. H., Hellem, T. L., Fiedler, K. K., Shi, X., Jeong, E. K., & Renshaw, P. F. (2011). Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. Journal of affective disorders, 135(1-3), 354-361. https://doi.org/10.1016/j.jad.2011.07.010
Kondo, D. G., Forrest, L. N., Shi, X., Sung, Y. H., Hellem, T. L., Huber, R. S., & Renshaw, P. F. (2016). Creatine target engagement with brain bioenergetics: a dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids, 48, 1941-1954. https://doi.org/10.1007/s00726-016-2194-3
Krahe, R., & Gabbiani, F. (2004). Burst firing in sensory systems. Nature Reviews Neuroscience, 5(1), 13–23. https://doi.org/10.1038/nrn1296
Leichsenring, F., Steinert, C., Rabung, S., & Ioannidis, J. P. A. (2022). The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World psychiatry : official journal of the World Psychiatric Association (WPA), 21(1), 133–145. https://doi.org/10.1002/wps.20941
Levental, U., Bersudsky, Y., Dwalatzky, T., Lerner, V., Medina, S., & Levine, J. (2015). A pilot open study of long term high dose creatine augmentation in patients with treatment resistant negative symptoms schizophrenia. Israel Journal of Psychiatry, 52(1), 6. https://pubmed.ncbi.nlm.nih.gov/25841104/
Lyoo, I. K., Yoon, S., Kim, T. S., Hwang, J., Kim, J. E., Won, W., Bae, S., & Renshaw, P. F. (2012). A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. The American journal of psychiatry, 169(9), 937–945. https://doi.org/10.1176/appi.ajp.2012.12010009
Mason, S. (2017). Lactate shuttles in neuroenergetics—Homeostasis, allostasis and beyond. Frontiers in Neuroscience, 11, 43. https://doi.org/10.3389/fnins.2017.00043
McMorris, T., Harris, R. C., Swain, J., Corbett, J., Collard, K., Dyson, R. J., Dye, L., Hodgson, C., & Draper, N. (2006). Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology, 185(1), 93–103. https://doi.org/10.1007/s00213-005-0269-z
McMorris, T., Harris, R. C., Howard, A. N., Langridge, G., Hall, B., Corbett, J., Dicks, M., & Hodgson, C. (2007). Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiology & Behavior, 90(1), 21–28. https://doi.org/10.1016/j.physbeh.2006.08.024
Miller, E. (2022, October 15). Metabolic pathways explained. Cleveland Clinic. https://health.clevelandclinic.org/metabolic-pathways-metabolic-conditioning
Munn, Z., Pollock, D., Khalil, H., Alexander, L., Mclnerney, P., Godfrey, C. M., Peters, M., & Tricco, A. C. (2022). What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI evidence synthesis, 20(4), 950–952. https://doi.org/10.11124/JBIES-21-00483
Nemets, B., & Levine, J. (2013). A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression. International clinical psychopharmacology, 28(3), 127-133. https://doi.org/10.1097/YIC.0b013e32835ff20f
Pan, J. W., & Takahashi, K. (2007). Cerebral energetic effects of creatine supplementation in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292(4), R1745-R1750. https://doi.org/10.1152/ajpregu.00717.2006
Peters, M. D., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., & Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. International journal of evidence-based healthcare, 13(3), 141–146. https://doi.org/10.1097/XEB.0000000000000050
Poortmans, J. R., & Francaux, M. (1999). Long-term oral creatine supplementation does not impair renal function in healthy athletes. Medicine and science in sports and exercise, 31(8), 1108-1110. https://doi.org/10.1097/00005768-199908000-00005
Prokopidis, K., Giannos, P., Triantafyllidis, K. K., Kechagias, K. S., Forbes, S. C., & Candow, D. G. (2023). Effects of creatine supplementation on memory in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. Nutrition reviews, 81(4), 416–427. https://doi.org/10.1093/nutrit/nuac064
Roitman, S., Green, T., Osher, Y., Karni, N., & Levine, J. (2007). Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disorders, 9(7), 754-758. https://doi.org/10.1111/j.1399-5618.2007.00532.x
Sherpa, N. N., De Giorgi, R., Ostinelli, E. G., Choudhury, A., Dolma, T., & Dorjee, S. (2025). Efficacy and safety profile of oral creatine monohydrate in add-on to cognitive-behavioural therapy in depression: An 8-week pilot, double-blind, randomised, placebo-controlled feasibility and exploratory trial in an under-resourced area. European Neuropsychopharmacology, 90, 28-35.https://doi.org/10.1016/j.euroneuro.2024.10.004
Smith, A. N., Morris, J. K., Carbuhn, A. F., Keller, J. E., Sullivan, D. K., & Taylor, M. K. (2023). Creatine as a therapeutic target in Alzheimer's disease. Current Developments in Nutrition, 7(11), 102011. https://doi.org/10.1016/j.cdnut.2023.102011
Sullivan, P. G., Geiger, J. D., Mattson, M. P., & Scheff, S. W. (2000). Dietary supplement creatine protects against traumatic brain injury. Annals of neurology, 48(5), 723–729. https://pubmed.ncbi.nlm.nih.gov/11079535/
Toniolo, R. A., Fernandes, F. B. F., Silva, M., Dias, R. D. S., & Lafer, B. (2017). Cognitive effects of creatine monohydrate adjunctive therapy in patients with bipolar depression: Results from a randomized, double-blind, placebo-controlled trial. Journal of affective disorders, 224, 69–75. https://doi.org/10.1016/j.jad.2016.11.029
Toniolo, R. A., Silva, M., Fernandes, F. D. B. F., Amaral, J. A. D. M. S., Dias, R. D. S., & Lafer, B. (2018). A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression. Journal of Neural Transmission, 125, 247-257. https://doi.org/10.1007/s00702-017-1817-5
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., Lewin, S., … Straus, S. E. (2018). PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Annals of internal medicine, 169(7), 467–473. https://doi.org/10.7326/M`18-0850
Turner, C. E., Byblow, W. D., & Gant, N. (2015). Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. The Journal of Neuroscience, 35(4), 1773–1780. https://doi.org/10.1523/JNEUROSCI.3113-14.2015
Vittengl, J. R., Clark, L. A., Smits, J. A., Thase, M. E., & Jarrett, R. B. (2019). Do comorbid social and other anxiety disorders predict outcomes during and after cognitive therapy for depression? Journal of Affective Disorders, 242, 150–158. https://doi.org/10.1016/j.jad.2018.08.036
Walsh, B., Tonkonogi, M., Söderlund, K., Hultman, E., Saks, V., & Sahlin, K. (2001). The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. The Journal of physiology, 537(3), 971-978.https://doi.org/10.1111/j.1469-7793.2001.00971.x
World Health Organization (WHO). (2022). Mental disorders Fact Sheet. Retrieved March 3, 2025 from https://www.who.int/news-room/fact-sheets/detail/mental-disorders
Xu, C., Bi, S., Zhang, W., & Luo, L. (2024). The effects of creatine supplementation on cognitive function in adults: A systematic review and meta-analysis. Frontiers in Nutrition, 11. https://doi.org/10.3389/fnut.2024.1424972
Yang, L., Calingasan, N. Y., Wille, E. J., Cormier, K., Smith, K., Ferrante, R. J., & Flint Beal, M. (2009). Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. Journal of neurochemistry, 109(5), 1427-1439. https://doi.org/10.1111/j.1471-4159.2009.06074.x
Yoon, S., Kim, J. E., Hwang, J., Kim, T. S., Kang, H. J., Namgung, E., Ban, S., Oh, S., Yang, J., Renshaw, P. F., & Lyoo, I. K. (2016). Effects of creatine monohydrate augmentation on brain metabolic and network outcome measures in women with major depressive disorder. Biological Psychiatry, 80(6), 439–447. https://doi.org/10.1016/j.biopsych.2015.11.027