Episode 167: Long-Acting Injectables with Dr. Cummings
By listening to this episode, you can earn 1 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Vardaan Bhat, Michael Cummings, MD, David Puder, MD
None of the authors have any conflicts of interest
In today’s episode of the podcast, we discuss the use of long-acting injectable (LAI) antipsychotics. LAIs are administered in intervals ranging from every 2 weeks to every 6 months, eliminating the need for daily oral antipsychotics and thereby improving adherence.
When Are Long-Acting Injectables Helpful?
The most notable advantage of LAIs is improved adherence compared to oral formulations. In Europe, 40-50% of patients experiencing psychosis are treated with LAIs (Watts, 2014). In the U.S., the rate can be as low as 10% of treatments (Kane et al., 2021). This is unfortunate, as one of the main reasons for relapses is non-adherence to the oral medications (Robinson et al., 1999; Caseiro et al., 2012). For oral medications, adherence rates are extremely low, often approaching ~1/3, even including a ‘fudge factor’ (classifying 80% adherence as adherent) (Valenstein et al., 2006). There are a number of factors that might contribute to this, including adverse effects and impaired insight into one’s own symptoms (anosognosia).
A benefit to injectables is they do not require a daily adherence schedule. There are formulations of paliperidone palmitate that can be administered as infrequently as every three or six months. Additionally, noncompliance may be less of a concern with LAIs since they cannot be hidden (“cheeked”) or spit out.
A number of studies demonstrate LAIs are associated with lower rates of relapse, hospitalization, and mortality. Perhaps most notably, a Swedish study by Taipale et al. of 29,883 birth-death records showed that, over the course of 7 years, those taking LAIs had a 33% lower rate of all-cause mortality (including death by suicide, accident, overdose, violence) than those taking the equivalent oral counterparts (0.67, 0.56–0.80) (Taipale et al., 2018).
Injection Tips
It is important for psychiatrists to know how to administer these medications to their patients. Adherence is more likely when patients do not have to make a third-party appointment with a provider they may or may not trust.
Nurses deliver injections regularly and can teach this skill very quickly. For the saline-based LAIs, the injection is usually straight in the gluteal or deltoid muscle. The needle goes in and is drawn back slightly (to make sure it’s not in a blood vessel), injected, and pulled out.
For first generation oil-based LAIs such as haloperidol decanoate and fluphenazine decanoate, the Z-track method is used, which involves pulling the skin & subcutaneous tissue while injecting and releasing them afterwards. This prevents the needle from leaving a continuous passageway from muscle to subcutaneous tissue, sealing the medication in the muscle. It leaves behind a viscous liquid material that slowly releases medication into the bloodstream over time. Many of these injectable medications last for a month or more, depending on the drug.
Figure 1: Z-track technique (illustrated)
Note: from Shepherd E (2018) Injection technique 1: administering drugs via the intramuscular route. Nursing Times [online]; 118: 4, 23-25. This article was updated by Shepherd E on 9 March 2022. https://www.nursingtimes.net/clinical-archive/assessment-skills/injection-technique-1-administering-drugs-via-the-intramuscular-route-2-15-03-2022/
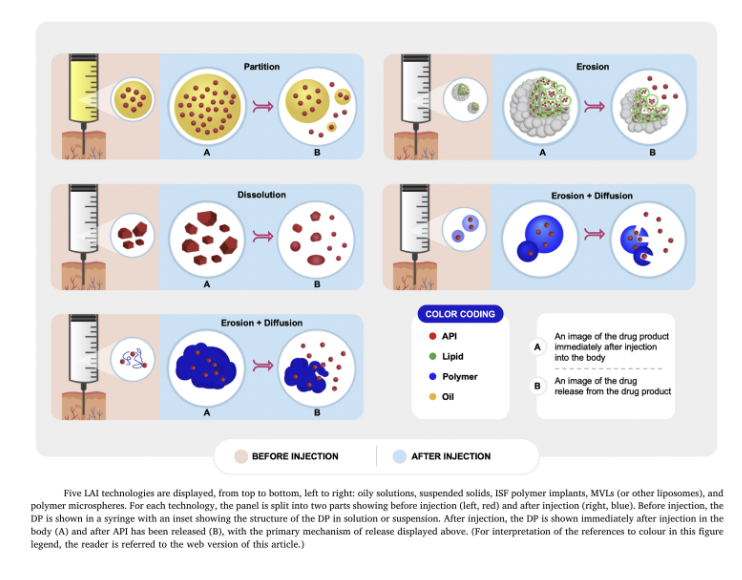
How LAIs Work
Figure 2: Ester LAI antipsychotic disposition
Note. from O'Brien, M. N., Jiang, W., Wang, Y., & Loffredo, D. M. (2021). Challenges and opportunities in the development of complex generic long-acting injectable drug products. Journal of Controlled Release, 336, 144–158. https://doi.org/10.1016/j.jconrel.2021.06.017
Decanoates are bonded to a 10-carbon lipid chain that is suspended in sesame oil, which is injected into the belly of the muscle where it forms a small sphere and gradually releases the medication. In fluphenazine’s case, the most common dose interval is 2 weeks. For haloperidol, the dose interval is usually 4 weeks.
With drugs like paliperidone palmitate, a microcrystal suspended in saline is injected into the muscle, where it slowly dissolves. The reason they last a month is that the tiny crystals dissolve quickly and release the medication early, while the bigger crystals dissolve more slowly and provide the later dosing of the drug. Formulations of paliperidone palmitate with even larger ranges of crystal sizes last three and six months, respectively.
There is also a version of depot risperidone with microspheres, similar to crystals, that is bonded to a polymer that dissolves.
Two LAI formulations of aripiprazole include aripiprazole monohydrate, which is a crystal, and aripiprazole lauroxil, which is a different type of crystal and has longer variability in terms of how slowly it can dissolve.
There is an LAI formulation of olanzapine pamoate that is bonded to a salt crystal. One version is dosed every 2 weeks and another is dosed every 4 weeks.
In short, the idea is that once these medications are in the muscle, they stay there and, by one mechanism or another, slowly release the drug into the bloodstream.
When Are LAIs Not Useful?
LAIs are not contraindicated in any situation where an antipsychotic is appropriate to use. It is advisable to wait to start an LAI until it is certain the person responds to the medication and tolerates it well.
The biggest caveat is that the elimination half-life is incredibly long. If an LAI is administered and it does not work well, the patient will have to wait 5 half-lives. In the longest case, Invega Hafyera (paliperidone palmitate), designed to last 6 months, will require 30 months for wash-out. Others have half lives from 21-46.5 days, so wash-out is still very long.
Risks
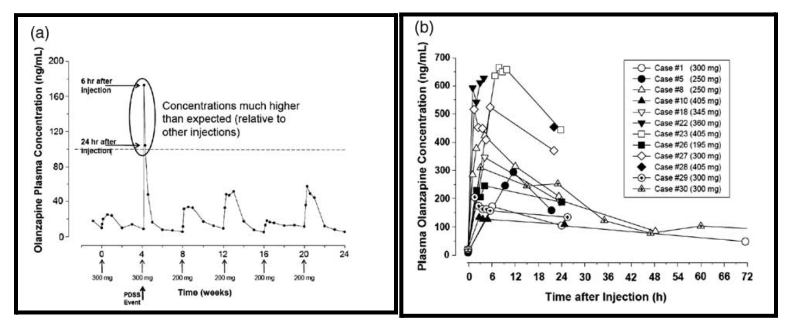
Unlike the others, olanzapine has a small but nonzero (0.1%) risk of rapid release. The FDA requires that patients be watched for three hours post administration to monitor for sedation via rapid release.
If the olanzapine LAI does release rapidly and a patient becomes very sedated, they should be observed until they wake back up and have their vitals taken. They’ve essentially received an overdose of the drug.
Because of the rapid-release warning, olanzapine pamoate is not often used. The risk of rapid dissociation doesn’t exist for any of the other LAIs.
Figure 3: “Kinetic profiles of severe sedation cases with olanzapine pamoate. (a) Single detailed case example, (b) multiple examples.” (Meyer, 2013)
Note. from Meyer, J. M. (2013). Understanding depot antipsychotics: An illustrated guide to kinetics. CNS Spectrums, 18(s1), 55–68. https://doi.org/10.1017/s1092852913000783
Side Effects of LAIs vs. Oral Counterparts
It is suggested that LAIs may have comparable or improved tolerability relative to their oral counterparts, since LAIs lack the sharp peak plasma concentration & peak-trough ratio seen in oral medications.
Transitioning From Oral to LAI
Switching from oral to long-acting injectable (LAI) antipsychotics may require a loading dose to reach steady state more quickly. The specific loading and maintenance doses vary depending on the LAI being used.
Haloperidol decanoate:
Such a slow climb that if started and given every month, it would take 3-5 months to reach steady state.
This is overcome by loading the drug. E.g., switching from oral 20 mg haloperidol daily and loading it at 200 mg/week for 3 weeks.
The oral medication can be stopped when the second loading dose is given. The maintenance dose can be started 14 days after the second loading dose, then 20x original dosing once a month. E.g., 400 mg haloperidol once a month, usually administered 200 mg every 2 weeks due to volume issues.
Fluphenazine decanoate:
Very similar to haloperidol decanoate.
Usually loaded at 25-50 mg a week for 3 weeks and continued at a maintenance dose starting 2 weeks after the last loading dose.
10 mg a day orally ~= 12.5-25 mg fluphenazine decanoate.
Paliperidone palmitate:
The starting point is a monthly dose (INVEGA SUSTENNA) because the INVEGA TRINZA (every 3 months) and INVEGA HAFYERA (every 6 month) are intended for people who have already responded to and are stable on the monthly injection (due to extended washout from longer-acting formulations).
Usually start with 156 mg or 234 mg as the initial injection.
1 week later, depending on target blood levels, another dose of either 156/234 mg
Then simply continue every month.
One limitation:
234 mg/month is equivalent to ~4.5-5.5mg/day oral risperidone.
If a patient required >4.5-5.5mg/day oral risperidone, they may have difficulty using INVEGA SUSTENNA because the corresponding LAI dosage can’t be achieved within the recommended dose range.
The number of doses can be increased beyond the recommended dose range, but this becomes prohibitively expensive because of the proprietary nature of the drug.
● Cost comparison:
Haloperidol decanoate and fluphenazine decanoate have a wholesale cost of less than $500 per year.
The wholesale acquisition cost at max dose for INVEGA SUSTENNA is ~$25,000 per year.
Risperidone (2 LAI formulations):
RISPERDAL CONSTA (with oral crossover for 3 weeks, as it doesn’t start dissolving until then)
Injected every 2 weeks
RISPERIDONE PERSERIS (subcutaneous formulation): comes in 90 mg and 120 mg doses
Roughly equal to 3-4 mg/day oral Risperidone
Doesn’t require oral crossover.
Aripiprazole (2 LAI formulations):
Aripiprazole monohydrate/ABILIFY MAINTENA:
Give an initial injection of 300-400 mg and continue half the oral dose for 2 weeks.
In a lot of countries outside the US, they’ve replaced this with a second injection after 1 week, followed by maintenance treatment.
Aripiprazole lauroxil/ARISTADA INITIO:
Give an initial dose of ARISTADA INITIO (rapid-release formulation of ARISTADA) and then start the maintenance medication a week later.
Doesn’t require oral crossover.
Olanzapine pamoate (ZYPREXA RELPREVV):
No oral crossover required
Two possible dosing intervals: once every 2 weeks and once every 4 weeks
Note risk of rapid release, as discussed under “Risks”
Metabolic Dysregulation
A meta-analysis by Pillinger et al. in 2019 looked at comparative metabolic changes from taking 18 antipsychotics combining 100 randomized trials in 25,952 patients for predictors of metabolic dysregulation and associations. In regards to weight change, ziprasidone and haloperidol were lower risk whereas quetiapine, olanzapine and clozapine were high risk.
Figure 4: Forest plots of glucose and weight change in patients using antipsychotics
Note. from Pillinger, T., McCutcheon, R. A., Vano, L., Mizuno, Y., Arumuham, A., Hindley, G., Beck, K., Natesan, S., Efthimiou, O., Cipriani, A., & Howes, O. D. (2020). Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. The Lancet Psychiatry, 7(1), 64–77. https://doi.org/10.1016/s2215-0366(19)30416-x
Limitations of ziprasidone are that it is not available as an LAI, it is difficult for GI tract to absorb, and has to be taken proximate (within 30 minutes) of a 500 calorie meal; otherwise, absorption is 50% or less of what it would be. Also, looking at other meta analyses, ziprasidone tends to end up at or near the bottom in terms of efficacy.
Managing Metabolic Syndrome And Weight Gain
It is suggested that psychiatrists have not been nearly aggressive enough in intervening to prevent or treat metabolic syndrome.
Criteria for proactive metabolic treatment:
Family/personal history of obesity
Weight gain of:
5% or more in first month of antipsychotic use
7.5% in first 2 months
10% (or increase in BMI of 1) in first 6 months
Most common preventive agent against metabolic syndrome:
Metformin
Increases insulin sensitivity
Not very prone to cause hypoglycemia
Does a very nice job of preventing AP-associated weight gain and glucose intolerance.
If someone has already gained weight and they are already glucose intolerant, the metformin ceases to be very effective.
A new class of med being used in circumstances where it’s not appropriate to switch antipsychotics is the glucagon-like peptide-1 (GLP-1) receptor agonists.
Liraglutide is the most studied in psychiatry.
Several GLP-1 agonists available on the US market:
6 injections, 1 oral form.
In most studies, ~⅔ of people achieve normal glucose tolerance.
People also tend to lose about 10-30% of baseline body weight.
The future for GLP-1 agonists in psychiatry looks bright. At the moment, metformin can help prevent metabolic syndrome but doesn’t reverse it. GLP-1 agonists may offer a solution.
(Note: Episode 158 with Rocio Salas-Whalen, an endocrinologist who focuses on weight management, delves further into the literature on GLP-1 agonists.)
One particularly notable study shows 50% of patients achieving 20% weight loss in a year on tirzepatide, which is extremely promising (Jastreboff et al., 2022).
Side Effects Of GLP-1 Agonists
Side effects of GLP-1 antagonists include nausea, GERD, and vomiting (largely because they delay gastric emptying and the stomach feels very full). Patients need to decrease what they eat, especially at night. As most of these medications are titrated from a lower dose to a higher dose the nausea and GI effects tend to mitigate over time. GLP-1 agonists may also suppress thirst, potentially leading to dehydration. As a result, it’s important to remind patients to keep drinking enough water.
Rare risks are pancreatitis and a theoretical possibility of pancreatic carcinoma based on hyperplasia. However, this is based on animal studies and has not yet been seen in humans. If the person being treated has not developed type 2 diabetes, the risk to the pancreas is much lower.
Another listed contraindication is for people with a family history of endocrine neoplasia syndrome type 2, a very rare genetic predisposition towards endocrine cancers. Most psychiatric doctors are not likely to run across many patients with that history.
LAIs During Pregnancy
A review of data on the safety profile of antipsychotics, including LAI formulations, suggests prescribing them during pregnancy is safe (Reinstein et al., 2020). In fact, women with psychotic disorders are more prone to relapse when pregnant (Taylor et al., 2018), so taking away their best defense against psychotic episodes makes them more likely to become psychotic and is not usually advisable.
Serious Mental Illness (SMI) and Homelessness
Unfortunately, every predictor of SMI can be found in the homeless. In countries such as Italy and Austria, SMI population are discharged to a supervised house/apartment where they can stay for up to a decade. In America, on the other hand, individuals with SMI are discharged to the street.
Mental illness appears to be a significant risk factor for homelessness; 49% of older homeless adults surveyed in Minnesota reported a serious mental illness (for instance, SAMHSA, 2011), and data also suggests that 75% of chronically homeless people struggle with substance use disorder (SUD) or an SMI (Streeter, 2022). In addition to expanded access to mental healthcare for homeless individuals, there needs to be a greater focus on stable and affordable housing, as most social programs seem to be insufficient if the person does not have stable housing.
Conclusion
Long-acting injectable antipsychotics appear effective at improving adherence, are comparably well-tolerated to other formulations, and decrease all-cause mortality. Nevertheless, they are used less than their oral counterparts in most settings. There are also a number of practical considerations, particularly with regards to drug kinetics, that providers should take into account when using LAIs.
As a society, we vastly undertreat the seriously mentally ill. LAIs are a tool we should consider using more in this population to help break the vicious cycle of hospitalization, discharge (without support/supervision), decompensation, and homelessness or incarceration.
References:
Caseiro, O., Pérez-Iglesias, R., Mata, I., Martínez-Garcia, O., Pelayo-Terán, J. M., Tabares-Seisdedos, R., Ortiz-García de la Foz, V., Vázquez-Barquero, J. L., & Crespo-Facorro, B. (2012). Predicting relapse after a first episode of non-affective psychosis: A three-year follow-up study. Journal of Psychiatric Research, 46(8), 1099–1105. https://doi.org/10.1016/j.jpsychires.2012.05.001
Huybrechts, K. F., Hernández-Díaz, S., Patorno, E., Desai, R. J., Mogun, H., Dejene, S. Z., Cohen, J. M., Panchaud, A., Cohen, L., & Bateman, B. T. (2016). Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry, 73(9), 938. https://doi.org/10.1001/jamapsychiatry.2016.1520
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., & Stefanski, A. (2022). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205–216. https://doi.org/10.1056/nejmoa2206038
Kane, J. M., McEvoy, J. P., Correll, C. U., & Llorca, P.-M. (2021). Controversies surrounding the use of long-acting injectable antipsychotic medications for the treatment of patients with schizophrenia. CNS Drugs, 35(11), 1189–1205. https://doi.org/10.1007/s40263-021-00861-6
Puder, D. (Host), Salas-Whalen, R. (Guest). (2022, September 23). Obesity and Weight Loss with Endocrinologist Rocio Salas-Whalen (Ep. 158) [Audio podcast episode]. In Psychiatry & Psychotherapy Podcast.https://www.psychiatrypodcast.com/psychiatry-psychotherapy-podcast/episode-158-obesity-and-weight-loss-with-endocrinologist-rocio-salas-whalen
Reinstein, S. A., Cosgrove, J., Malekshahi, T., & Deligiannidis, K. M. (2020). Long-acting injectable antipsychotic use during pregnancy. The Journal of Clinical Psychiatry, 81(6). https://doi.org/10.4088/jcp.20ac13597
Robinson, D., Woerner, M. G., Alvir, J. M., Bilder, R., Goldman, R., Geisler, S., Koreen, A., Sheitman, B., Chakos, M., Mayerhoff, D., & Lieberman, J. A. (1999). Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of General Psychiatry, 56(3), 241. https://doi.org/10.1001/archpsyc.56.3.241
SAMHSA (2011, July).Current Statistics on the Prevalence and Characteristics of People Experiencing Homelessness in the United States. Substance Abuse and Mental Health Services Administration. Retrieved January 6, 2023, from https://www.samhsa.gov/sites/default/files/programs_campaigns/homelessness_programs_resources/hrc-factsheet-current-statistics-prevalence-characteristics-homelessness.pdf
Shepherd, E. (2018). Injection technique 1: administering drugs via the intramuscular route. Nursing Times, 114(8), 23–25. Retrieved January 8, 2023, from https://www.nursingtimes.net/clinical-archive/assessment-skills/injection-technique-1-administering-drugs-via-the-intramuscular-route-2-15-03-2022/
Streeter, J. L. (2022, May). Homelessness in California: Causes and policy considerations. Stanford Institute for Economic Policy Research (SIEPR). Retrieved January 6, 2023, from https://siepr.stanford.edu/publications/policy-brief/homelessness-california-causes-and-policy-considerations
Taipale, H., Mittendorfer-Rutz, E., Alexanderson, K., Majak, M., Mehtälä, J., Hoti, F., Jedenius, E., Enkusson, D., Leval, A., Sermon, J., Tanskanen, A., & Tiihonen, J. (2018). Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophrenia Research, 197, 274–280. https://doi.org/10.1016/j.schres.2017.12.010
Taylor CL, Broadbent M, Khondoker M, et al. Predictors of severe relapse in pregnant women with psychotic or bipolar disorders. J Psychiatr Res. 2018;104:100–107.
Valenstein, M., Ganoczy, D., McCarthy, J. F., Kim, H. M., Lee, T. A., & Blow, F. C. (2006). Antipsychotic adherence over time among patients receiving treatment for schizophrenia. The Journal of Clinical Psychiatry, 67(10), 1542–1550. https://doi.org/10.4088/jcp.v67n1008
Watts, V. (2014). Some experts urge more use of long-acting, injectable antipsychotics. Psychiatric News, 49(23), 1–1. https://doi.org/10.1176/appi.pn.2014.12a8
Supplementary Readings:
Bakker, I. C., Schubart, C. D., & Zelissen, P. M. (2016). Successful treatment of a prolactinoma with the antipsychotic drug aripiprazole. Endocrinology, Diabetes & Metabolism Case Reports, 2016. https://doi.org/10.1530/edm-16-0028
Chung, Y., & Cannon, T. D. (2015). Brain imaging during the transition from psychosis prodrome to schizophrenia. Journal of Nervous & Mental Disease, 203(5), 336–341. https://doi.org/10.1097/nmd.0000000000000286
Correll, C. U., Rubio, J. M., & Kane, J. M. (2018). What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry, 17(2), 149–160. https://doi.org/10.1002/wps.20516
Dietsche, B., Kircher, T., & Falkenberg, I. (2017). Structural brain changes in schizophrenia at different stages of the illness: A selective review of Longitudinal Magnetic Resonance Imaging Studies. Australian & New Zealand Journal of Psychiatry, 51(5), 500–508. https://doi.org/10.1177/0004867417699473
Drake, R. J., Husain, N., Marshall, M., Lewis, S. W., Tomenson, B., Chaudhry, I. B., Everard, L., Singh, S., Freemantle, N., Fowler, D., Jones, P. B., Amos, T., Sharma, V., Green, C. D., Fisher, H., Murray, R. M., Wykes, T., Buchan, I., & Birchwood, M. (2020). Effect of delaying treatment of first-episode psychosis on symptoms and social outcomes: A longitudinal analysis and Modelling Study. The Lancet Psychiatry, 7(7), 602–610. https://doi.org/10.1016/s2215-0366(20)30147-4
Díaz, I., Pelayo-Terán, J. M., Pérez-Iglesias, R., Mata, I., Tabarés-Seisdedos, R., Suárez-Pinilla, P., Vázquez-Barquero, J. L., & Crespo-Facorro, B. (2013). Predictors of clinical remission following a first episode of non-affective psychosis: Sociodemographics, premorbid and clinical variables. Psychiatry Research, 206(2-3), 181–187. https://doi.org/10.1016/j.psychres.2012.10.011
Fang, S.-C., Huang, C.-Y., & Shao, Y.-H. J. (2022). Long-term outcomes of early use of long-acting injectable antipsychotics in Schizophrenia. The Journal of Clinical Psychiatry, 83(4). https://doi.org/10.4088/jcp.21r14153
FDA. (2015, March 23). FDA Drug Safety Communication: FDA review of study sheds light on two deaths associated with the injectable schizophrenia drug Zyprexa Relprevv (olanzapine pamoate). U.S. Food and Drug Administration. Retrieved December 26, 2022, from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-study-sheds-light-two-deaths-associated-injectable
Gault, J. M., Davis, R., Cascella, N. G., Saks, E. R., Corripio-Collado, I., Anderson, W. S., Olincy, A., Thompson, J. A., Pomarol-Clotet, E., Sawa, A., Daskalakis, Z. J., Lipsman, N., & Abosch, A. (2017). Approaches to neuromodulation for schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry, 89(7), 777–787. https://doi.org/10.1136/jnnp-2017-316946
Goff, D. C., Falkai, P., Fleischhacker, W. W., Girgis, R. R., Kahn, R. M., Uchida, H., Zhao, J., & Lieberman, J. A. (2017). The long-term effects of antipsychotic medication on clinical course in schizophrenia. American Journal of Psychiatry, 174(9), 840–849. https://doi.org/10.1176/appi.ajp.2017.16091016
Hayes, R. D., Downs, J., Chang, C.-K., Jackson, R. G., Shetty, H., Broadbent, M., Hotopf, M., & Stewart, R. (2014). The effect of clozapine on premature mortality: An assessment of clinical monitoring and other potential confounders. Schizophrenia Bulletin, 41(3), 644–655. https://doi.org/10.1093/schbul/sbu120
Ho, B.-C., Andreasen, N. C., Ziebell, S., Pierson, R., & Magnotta, V. (2011). Long-term antipsychotic treatment and brain volumes. Archives of General Psychiatry, 68(2), 128. https://doi.org/10.1001/archgenpsychiatry.2010.199
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., Arndt, T., Bäckers, L., Rothe, P., Cipriani, A., Davis, J., Salanti, G., & Leucht, S. (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. The Lancet, 394(10202), 939–951. https://doi.org/10.1016/s0140-6736(19)31135-3
Hunsberger, J., Austin, D. R., Henter, I. D., & Chen, G. (2009). The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues in Clinical Neuroscience, 11(3), 333–348. https://doi.org/10.31887/dcns.2009.11.3/jhunsberger
Jayaram, M., Rattehalli, R. D., & Adams, C. E. (2012). Where does evidence from new trials for schizophrenia fit with the existing evidence: A case of the emperor's new clothes? Schizophrenia Research and Treatment, 2012, 1–4. https://doi.org/10.1155/2012/625738
Larsen, J. R., Vedtofte, L., Jakobsen, M. S., Jespersen, H. R., Jakobsen, M. I., Svensson, C. K., Koyuncu, K., Schjerning, O., Oturai, P. S., Kjaer, A., Nielsen, J., Holst, J. J., Ekstrøm, C. T., Correll, C. U., Vilsbøll, T., & Fink-Jensen, A. (2017). Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder. JAMA Psychiatry, 74(7), 719. https://doi.org/10.1001/jamapsychiatry.2017.1220
Lauriello, J., & Campbell, A. R. (2022, August 18). Pharmacotherapy for schizophrenia: Long-acting injectable antipsychotic drugs. UpToDate. Retrieved December 26, 2022, from https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-long-acting-injectable-antipsychotic-drugs
Lei, W., Kirkpatrick, B., Wang, Q., Deng, W., Li, M., Guo, W., Liang, S., Li, Y., Zhang, C., Li, X., Zhang, P., Li, Z., Xiang, B., Chen, J., Hu, X., Zhang, N., & Li, T. (2019). Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Research: Neuroimaging, 288, 12–20. https://doi.org/10.1016/j.pscychresns.2019.04.009
Leucht, S., Cipriani, A., Spineli, L., Mavridis, D., Örey, D., Richter, F., Samara, M., Barbui, C., Engel, R. R., Geddes, J. R., Kissling, W., Stapf, M. P., Lässig, B., Salanti, G., & Davis, J. M. (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. The Lancet, 382(9896), 951–962. https://doi.org/10.1016/s0140-6736(13)60733-3
MacEwan, J. P., Kamat, S. A., Duffy, R. A., Seabury, S., Chou, J. W., Legacy, S. N., Hartry, A., Eramo, A., & Karson, C. (2016). Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatric Services, 67(11), 1183–1188. https://doi.org/10.1176/appi.ps.201500455
Majer, I. M., Gaughran, F., Sapin, C., Beillat, M., & Treur, M. (2015). Efficacy, tolerability, and safety of Aripiprazole once-monthly versus other long-acting injectable antipsychotic therapies in the maintenance treatment of schizophrenia: A mixed treatment comparison of double-blind randomized clinical trials. Journal of Market Access & Health Policy, 3(1), 27208. https://doi.org/10.3402/jmahp.v3.27208
Meyer, J. M. (2013). Understanding depot antipsychotics: An illustrated guide to kinetics. CNS Spectrums, 18(s1), 55–68. https://doi.org/10.1017/s1092852913000783
Mikell, C. B., Sinha, S., Sheth, S. A. (2016). Neurosurgery for schizophrenia: An update on pathophysiology and a novel therapeutic target. Journal of Neurosurgery, 124(4), 917–928. https://doi.org/10.3171/2015.4.jns15120
Mohr, P., Knytl, P., Voráčková, V., Bravermanová, A., & Melicher, T. (2017). Long-acting injectable antipsychotics for prevention and management of violent behaviour in psychotic patients. International Journal of Clinical Practice, 71(9). https://doi.org/10.1111/ijcp.12997
Mortlock, A.-M., Larkin, F., Ross, C. C., Gupta, N., Sengupta, S., & Das, M. (2017). Effectiveness of paliperidone depot injection in seriously violent men with comorbid schizophrenia and dissocial personality disorder in a UK high-security hospital. Therapeutic Advances in Psychopharmacology, 7(5), 169–179. https://doi.org/10.1177/2045125317693513
Moseley, P., Alderson-Day, B., Ellison, A., Jardri, R., & Fernyhough, C. (2016). Non-invasive brain stimulation and auditory verbal hallucinations: New techniques and Future Directions. Frontiers in Neuroscience, 9. https://doi.org/10.3389/fnins.2015.00515
Ostuzzi, G., Bertolini, F., Del Giovane, C., Tedeschi, F., Bovo, C., Gastaldon, C., Nosé, M., Ogheri, F., Papola, D., Purgato, M., Turrini, G., Correll, C. U., & Barbui, C. (2021). Maintenance treatment with long-acting injectable antipsychotics for people with nonaffective psychoses: A network meta-analysis. American Journal of Psychiatry, 178(5), 424–436. https://doi.org/10.1176/appi.ajp.2020.20071120
Pillinger, T., McCutcheon, R. A., Vano, L., Mizuno, Y., Arumuham, A., Hindley, G., Beck, K., Natesan, S., Efthimiou, O., Cipriani, A., & Howes, O. D. (2020). Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. The Lancet Psychiatry, 7(1), 64–77. https://doi.org/10.1016/s2215-0366(19)30416-x
Salem, H., Nagpal, C., Pigott, T., & Teixeira, A. L. (2017). Revisiting antipsychotic-induced akathisia: Current issues and prospective challenges. Current Neuropharmacology, 15(5). https://doi.org/10.2174/1570159x14666161208153644
Schreiner, A., Bergmans, P., Cherubin, P., Keim, S., Llorca, P.-M., Cosar, B., Petralia, A., Corrivetti, G., & Hargarter, L. (2015). Paliperidone palmitate in non-acute patients with schizophrenia previously unsuccessfully treated with risperidone long-acting therapy or frequently used conventional depot antipsychotics. Journal of Psychopharmacology, 29(8), 910–922. https://doi.org/10.1177/0269881115586284
Shulman, M., Miller, A., Misher, J., & Tentler, A. (2014). Managing cardiovascular disease risk in patients treated with antipsychotics: A multidisciplinary approach. Journal of Multidisciplinary Healthcare, 489. https://doi.org/10.2147/jmdh.s49817
Tiihonen, J. (2012). Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Archives of General Psychiatry, 69(5), 476. https://doi.org/10.1001/archgenpsychiatry.2011.1532
Tiihonen, J., Tanskanen, A., & Taipale, H. (2018). 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. American Journal of Psychiatry, 175(8), 765–773. https://doi.org/10.1176/appi.ajp.2018.17091001
van Erp, T. G., Hibar, D. P., Rasmussen, J. M., Glahn, D. C., Pearlson, G. D., Andreassen, O. A., Agartz, I., Westlye, L. T., Haukvik, U. K., Dale, A. M., Melle, I., Hartberg, C. B., Gruber, O., Kraemer, B., Zilles, D., Donohoe, G., Kelly, S., McDonald, C., Morris, D. W., … Turner, J. A. (2015). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the Enigma consortium. Molecular Psychiatry, 21(4), 547–553. https://doi.org/10.1038/mp.2015.63
Wiles, D. H., McCreadie, R. G., & Whitehead, A. (1990). Pharmacokinetics of haloperidol and fluphenazine decanoates in chronic schizophrenia. Psychopharmacology, 101(2), 274–281. https://doi.org/10.1007/bf02244140
Wilson, W. H. (2004). A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. Journal of Psychiatric Practice, 10(6), 393–401. https://doi.org/10.1097/00131746-200411000-00009
Yung, A. R., & Nelson, B. (2011). Young people at ultra high risk for psychosis: Research from the Pace Clinic. Revista Brasileira De Psiquiatria, 33(suppl 2). https://doi.org/10.1590/s1516-44462011000600003
Zivkovic, S., Koh, C. H., Kaza, N., & Jackson, C. A. (2019). Antipsychotic drug use and risk of stroke and myocardial infarction: A systematic review and meta-analysis. BMC Psychiatry, 19(1). https://doi.org/10.1186/s12888-019-2177-5