Episode 165: Exercise for the Brain
By listening to this episode, you can earn 0.75 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Sonia Ann Marie F. Dela Cruz, MD, Sarah Alam, MD, Manojna Kintada, DO, Derek Lanuto, MD , David Puder, MD
There are no conflicts of interest for this episode.
The Muscle as an Endocrine Organ
Exercise is an integral contributor to brain health. Physical activity slows the rate of cognitive decline in healthy people and in people with neurodegenerative disorders throughout their lifespan (Severinsen, 2020). It also has beneficial effects on mood, sleep, appetite, learning, memory, and executive function (Severinsen, 2020). In fact, exercise has been shown to increase hippocampal volume and blood flow, as well as induce neurogenesis in the dentate gyrus and increase synaptic plasticity (Severinsen, 2020).
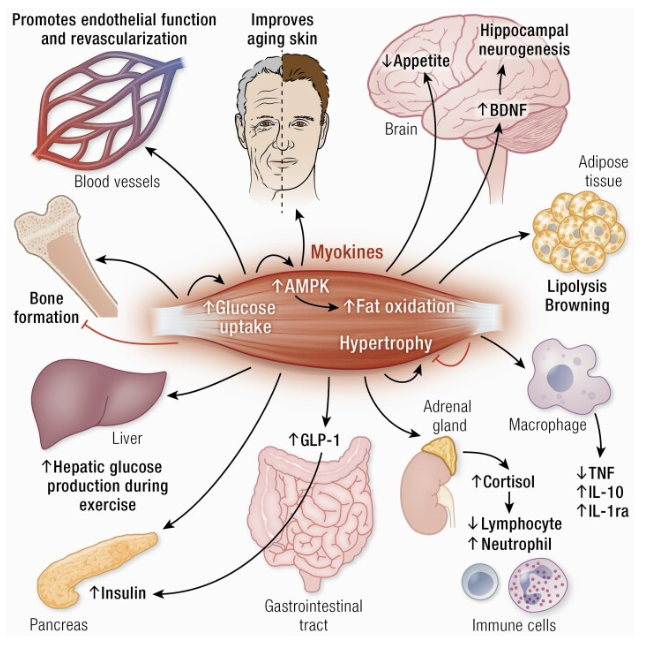
In attempts to elucidate the pathophysiology behind such positive effects of exercise, an interesting idea that emerged within the past couple of decades was the concept of contracting skeletal muscle behaving as an endocrine organ. Upon contraction, muscles release “myokines” (first termed in 2003), which are cytokines and peptides that mediate communication with other organs such as the brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, skin and muscle itself (Oudbier, 2022). They affect cognition, lipid and glucose metabolism, muscular hypertrophy and bone formation, to name a few systems (see Figure 1). Myokines can be both pro-inflammatory and anti-inflammatory (Oudbier, 2022). Examples include IL-6, IL-8, IL-15 and brain derived neurotrophic factor (BDNF) (Pedersen, 2008). BDNF is a growth factor for the hippocampus that is involved in cell survival, learning and neurogenesis. Muscle contractions lead to the secretion of myokines cathepsin-B and irisin which cross the blood–brain barrier to indirectly increase BDNF (Oudbier, 2022).
Figure 1. Effect of Myokines on Various Body Systems
Note. From “Muscle–Organ Crosstalk: The Emerging Roles of Myokines” by M.C.K. Severinsen, 2020, Endocrine reviews, 41(4), 594–609. https://doi.org/10.1210/endrev/bnaa016. Copyright © Endocrine Society 2020.
The “Myokine Concept” states that physical inactivity suppresses the endocrine function of muscle, tilting the balance towards inflammation and increasing the risk of dementia and cognitive impairment (Oudbier, 2022). When muscle atrophy occurs, fast-twitch type II fibers are lost, which leads to a switch to slow-twitch type I fibers, thus triggering a change in myokine secretion and a pro-inflammatory state (Oudbier, 2022). In combination with muscle atrophy, excess adipose tissue drives IL-6 signaling towards “inflamm-aging” and neurodegeneration (Oudbier, 2022). In fact, chronic inflammation has been associated with a two- to three-fold increase in the systemic concentrations of cytokines and is a strong predictor of all-cause mortality and cardiovascular-disease-cause mortality in the elderly (Pedersen, 2008). The recommendation to patients of increasing exercise (i.e., muscle contraction) serves to counteract this phenomenon.
Exercise and Dementia
Prevalence of dementia/Cost of dementia
According to the World Health Organization, there are more than 10 million new cases of dementia every year, and currently more than 55 million people live with dementia worldwide (World Health, 2022). Dementia has significant economic implications with an estimated total global cost of dementia in the US as 1.3 trillion dollars, and that cost in 2030 is expected to surpass $2.8 trillion (World Health, 2022). These staggering numbers have led to research on potential treatment options for dementia. One potential treatment option for dementia is exercise, which may be explained further by understanding the pathophysiology associated with exercise and dementia along with potential treatment options.
Pathophysiology
The pathophysiology between exercise and dementia is still being studied with different proposed mechanisms. One six-month randomized control study found aerobic activity leading to improved cortical connectivity when compared with controls (Colcombe, 2004). Exercise may lead to improved memory function due to reversing hippocampal volume loss, leading to improvements in spatial memory (Erikson, 2011). Exercise has been proposed to decrease vascular risk factors associated with dementia (Ahlskog, 2011). Studies have shown improved learning capabilities due to activating genes correlated with mitochondrial function and synaptic plasticity (Stranahan, 2010). Greater cerebral white matter integrity has been linked to higher aerobic fitness and lower obesity rate (Marks, 2007). Low skeletal muscle has also been associated with cognitive impairment and dementia in older adults, which may be due to the effects of exercise on systemic inflammation, insulin metabolism, protein metabolism, and mitochondrial function (Oudbier, 2022). Therefore, the pathophysiology behind how exercise is related to dementia is multifactorial and may be related to cortical connectivity, hippocampus volume size, decreased vascular risk factors, improved skeletal muscle mass, and synaptic plasticity.
Exercise as a Treatment
Research has demonstrated that higher levels of physical activity have been associated with a reduced risk of Alzheimer’s disease (Buchman, 2012). One study found patients with mild to moderate cognitive impairment in randomized controlled trials had better cognitive scores after 6-12 months of exercise when compared with sedentary controls (Ahlskog, 2011). Exercise may also improve a patient’s ability to perform their ADLS. One study with 134 ambulatory patients from five nursing homes with mild to severe dementia found that a 1-hour simple exercise program led to significantly slower decline in ADL scores. Exercise has been found to have better compliance and less side effects when compared to pharmacological interventions, and may be used as a beneficial adjunct to traditional pharmacological methods (Ströhle, 2015).
Additionally, neuropsychiatric symptoms were found to be reduced in patients with mild Alzheimer’s disease when they engaged in a moderate-to-high intensity exercise program (Smith, 2010). Recommendations of the type and amount of physical activity in the older adults was studied and found that it should emphasize muscle-strength activity, reducing sedentary behaviors, and moderate-intensity aerobic activity (Nelson, 2007). These studies suggest exercise being used as a treatment option for dementia. It may be used as a tool by clinicians to mitigate those with high risk factors for dementia, and suggests that exercise may be used as a preventive measure (Rolland 2007).
Inflammation, Mitochondria, Exercise & Mental Health
Exercise and its beneficial effects on the body are innumerable. There have been multiple studies that demonstrate the beneficial effects of exercise on both psychological and physical health. However, with age, it becomes more difficult to exercise to the same intensity and frequency. This can occur due to physical limitations, sedentary lifestyles, or amotivation. Furthermore, physical inactivity can contribute to low skeletal mass which may have detrimental effects on our cognition.
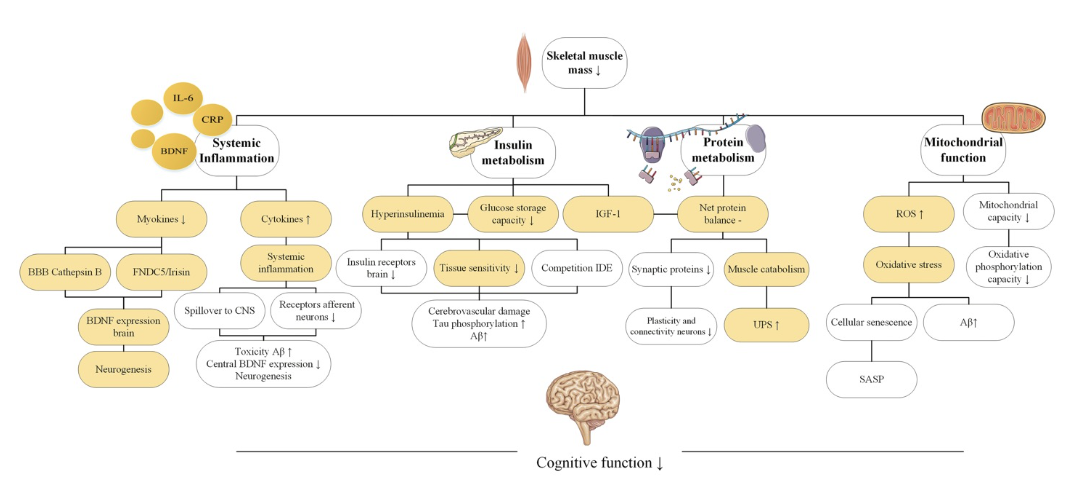
What is the link between low skeletal mass and cognition? In the narrative review, Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function, which was published in 2022, it emphasizes four primary pathophysiological mechanisms that may underlie the association between low skeletal mass and how it may contribute to a decline in cognition.
What are the four pathophysiological mechanisms proposed in this narrative review? In the narrative review, Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function, it focuses on four notable pathophysiological mechanisms. These mechanisms include systemic inflammation, protein metabolism, insulin metabolism, and mitochondrial function.
Before exploring these pathophysiological mechanisms on a deeper level, it is essential to understand low skeletal mass and cognitive dysfunction as one ages. During aging, there is a loss in skeletal muscle mass secondary to decrease in the number of muscle fibers and atrophy of the muscle fibers. Furthermore, it is also noted that during aging there is also a loss of skeletal muscle type II fibers prior to type I fibers. Muscle fiber atrophy leads to anabolic resistance, which is defined as suppression of protein synthesis in response to anabolic stimuli. In addition, there is also a decrease in protein synthesis with aging. This has been linked to a decrease in neural plasticity and connectivity between the neurons. Furthermore, as anabolic resistance builds and there is a decrease in protein synthesis, there is also an increase in muscle breakdown or catabolism, which leads to an increase in toxic metabolites such as UPS (ubiquitin-proteasome system) which further exacerbates the negative protein balance seen with advanced age.
How does aging affect cognition?
There are many studies that display the plethora of effects of aging on cognition. Aging is the largest risk factor for cognitive decline. Of note, aging can cause cognitive decline due to malfunctioning neuronal networks, loss of synaptic plasticity, and changes in the neuronal structure. On a molecular level, these changes consist of decrease in the number of axons, decrease in the number of dendritic spines, and morphologic changes in the dendrites which can greatly impact neuronal plasticity and neural networks. These microscopic changes can manifest macroscopically, as cognitive decline greatly impacts short- and long-term memory.
As discussed above, in the narrative review, Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function, it focuses on four notable pathophysiological mechanisms. These mechanisms include systemic inflammation, protein metabolism, insulin metabolism, and mitochondrial function.
Systemic Inflammation
Low skeletal muscle mass is associated with low-grade chronic inflammation.
“Immunosenescence” is defined as less potent immune function that results from aging.
Systemic inflammation occurs with aging as there is an increase in pro-inflammatory cytokines.
Furthermore, there is a decrease in myokines which decreases neuroprotective factors such as BDNF.
Therefore, an increase in proinflammatory cytokines in conjunction with a decrease in neuroprotective factors increase the permeability of the blood-brain barrier, making the brain more permeable to toxins. In addition, it decreases central BDNF expression, which decreases neurogenesis.
In conclusion, systemic inflammation results from impaired myokine secretion which may contribute to cognitive decline due to a decrease in neuroprotective factors and an increase in blood brain barrier permeability.
Protein Metabolism
Muscle catabolism exceeds protein synthesis with aging. This leads to a negative protein balance. This can alter the way proteins are folded, packed, maintained, and broken down.
Negative protein balance can contribute to low skeletal muscle mass. In addition, a negative protein balance can also lower protein concentrations in the brain, which may impact cognition. Furthermore, misfolded proteins have been observed in many types of dementia.
Low skeletal mass is also related to upregulation of ubiquitin proteasome system (UPS) which is essential for removing short-lived, damaged, or misfolded proteins.
However, with a negative protein balance, more proteins are being broken down than synthesized. This upregulates the UPS system to clear the damaged proteins. However, when the UPS system becomes overworked, this causes the damaged proteins to accumulate, which can further exacerbate symptoms.
It is important to note that the upregulation of the UPS system in relation to sarcopenia still requires further investigation.
Insulin Metabolism
Skeletal muscle is instrumental in glucose homeostasis.
Low skeletal mass can lead to insulin resistance.
Furthermore, insulin resistance increases with age.
Insulin resistance is an independent risk factor for cognitive decline.
One of the main energy sources for the brain is glucose. When there is an increase in glucose concentration in the peripheral tissue, insulin sensitivity and glucose regulation is negatively impacted.
Glucose and insulin both pass the blood-brain barrier. With insulin resistance and excess glucose in the blood, neurotransmitter expression and synaptic remodeling are greatly affected. Furthermore, excess insulin decreases the number of insulin receptors in the blood-brain barrier and, thus, attenuates insulin transport in the brain.
Prolonged hyperinsulinemia decreases tissue sensitivity to insulin, leading to neurotoxicity.
Neurotoxic effects that result from hyperinsulinemia and insulin resistance include tau phosphorylation, increase in reactive oxygen species and oxidative stress, and toxicity of AB plaques which can exacerbate cognitive decline.
Mitochondrial Function
Skeletal muscle uses ATP generated by mitochondrial oxidative phosphorylation.
Skeletal muscle utilizes oxygen and, as a result, produces reactive oxygen species (ROS).
When there is a buildup of ROS in conjunction with dysfunctional mitochondria, it can damage cells at a molecular level due to the toxic effects of ROS.
With aging, the mitochondrial oxidative phosphorylation capacity decreases. Furthermore, the mitochondrial DNA undergoes more mutations, which results in oxidative tissue damage. When skeletal muscle does not receive adequate ATP due to dysfunctional mitochondria, cellular apoptosis occurs which leads to loss of skeletal muscle.
Cellular apoptosis also triggers pro-inflammatory cytokines and matrix metalloproteinases (MMPs) which further induce transcription of inflammatory markers.
An increase in ROS and oxidative damage also contributes to an increase in AB aggregates, which further contributes to mitochondrial dysfunction.
However, further investigation is needed to better understand the link between mitochondrial dysfunction and cognitive decline.
Figure 2
Note: From Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function, by S.J. Oudbier, J. Goh, S.M.L.M. Looijaard, E.M. Reijnierse, E.M. Meskers, C.G.M., and Maier A.B., 2022, The journals of gerontology. Series A, Biological sciences and medical sciences, 77(10), 1959–1968. https://doi.org/10.1093/gerona/glac121. Copyright © The Journals of Gerontology 2022.
Exercise and its Beneficial Effects on Cognition
Physical activity has positive effects on cognition.
Physical activity has shown to increase synaptic plasticity and cognition.
Exercise increases central BDNF expression which is neuroprotective and supports neural networks.
Exercise also helps preserve skeletal muscle mass which is essential as one ages.
Furthermore, exercise improves mental health by decreasing depression and anxiety levels.
Exercise can help form new social networks and stay connected with the community.
Exercise can be motivating via goal-setting and helps individuals cultivate discipline, complete challenges, and set new goals.
Cardiorespiratory Fitness and its Relationship to All-Cause Mortality
Often when evaluating interventions and treatments in medicine, the ultimate end point to assess is, “But does it affect mortality?” Comparing different treatments in their effect on mortality can drive our protocols and resources. While, intrinsically, often one would expect people in greater physical fitness to have better health outcomes and lower mortality overall, it is just as important to look at the evidence of this potential relationship, and even more so, as it turns out, the question “What is its magnitude?” A retrospective cohort study by Madsanger et al., published in JAMA Network Open in 2018, set out to evaluate the relationship that different levels of cardiorespiratory fitness had with mortality. But first, a quick statistics refresher:
Absolute Risk is the likelihood that an event or outcome will occur in a group.
Relative Risk (RR), also known as Risk Ratio, is the ratio of the incidence of an outcome, developing a disease, or complication in the exposed group to the incidence of the outcome in the unexposed group. When relative risk is equal to 1, there is no association between exposure and disease. When relative risk is less than 1, exposure is associated with decreased disease occurrence. For rare diseases, odds ratio approximates relative risk (Fletcher, 2014).
Relative Risk Reduction (RRR) is the proportion or percentage of risk reduction attributable to the intervention as compared to a control. It is calculated as absolute risk reduction divided by risk of the unexposed group. Absolute risk reduction is the actual difference in risk attributable to the intervention as compared to a control. It is calculated as the risk of the control minus the risk of intervention.
The Odds Ratio (OR) is the measure showing the association of the outcome given a particular exposure compared to the presence of the outcome without the exposure at a given point of time. An OR > 1 means that the odds of the outcome are greater with the exposure group vs the control group. OR < 1 means the odds of the outcome are lesser in the exposure group than control, and OR = 1 means the exposure doesn’t affect the outcome (Szumilas, 2010).
The Hazard ratio (HR) compares the chance of a negative outcome occurring in one group to another over a period of time, such as mortality in a treatment group versus a control group. HR can be calculated by dividing the chance of an outcome occurring in one group versus the chance of the outcome occurring in the other/control. A HR < 1 means that the measured outcome happens less frequently in the first comparison group, a HR > 1 means that the outcome happens more frequently in the first comparison group, and if HR = 1 then the outcome occurs equally in both groups.
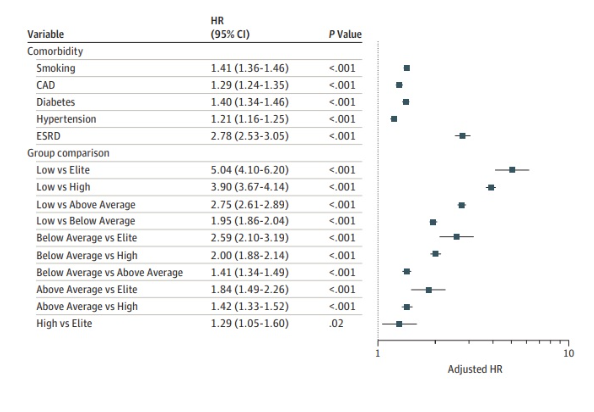
In the study by Madsanger et al. 2018 they compared long-term mortality by ANY cause among populations with different levels of cardiorespiratory fitness. Over a median time period of 8.4 years, they grouped the population into low performance, below average, above average, high, and elite. What they found was striking. They found that as fitness levels increased, risk of all-cause mortality decreased, without a limit. The elite group compared to the low fitness group showed a statistically significant HR of 0.2, indicating that over that period of time, the risk of death to ANY cause was 1/5 in the elite performance group compared to the low performance group. Even comparing the below average vs low fitness would have a RR of 0.51.
Compare these results to the relative risks shown by the treatment with statins to controls without statins in Byrne et al. 2022 that were between 0.91 and 0.86. Cardiorespiratory fitness clearly blows it away. Madsanger et al. even adjusted for and compared their results to the hazard ratios of other common health risk factors. Comparing the below average group to above average group had a HR of 1.41, which is comparable to the relative risk of smoking!
*Note. Reproduced from Figure 2C in Mandsager et al. 2018, showing hazard ratios for all-cause mortality adjusted for the most common medical risk factors.
This study is a strong argument for encouraging greater cardiorespiratory fitness in patients, as the results were shown to be dramatic and often more than the risk factors and conditions currently prioritized. The benefits were shown at all levels and therefore whether encouraging a patient to improve from low fitness to below average or an above average performer to strive for high performance, they can significantly improve their health and reduce their risk of mortality.
References (APA format):
Ahlskog, J. E., Geda, Y. E., Graff-Radford, N. R., & Petersen, R. C. (2011). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic proceedings, 86(9), 876–884. https://doi.org/10.4065/mcp.2011.0252
Buchman, A. S., Boyle, P. A., Yu, L., Shah, R. C., Wilson, R. S., & Bennett, D. A. (2012). Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology, 78(17), 1323–1329. https://doi.org/10.1212/WNL.0b013e3182535d35
Byrne, P., Demasi, M., Jones, M., Smith, S. M., O'Brien, K. K., & DuBroff, R. (2022). Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-analysis. JAMA internal medicine, 182(5), 474–481. https://doi.org/10.1001/jamainternmed.2022.0134
Colcombe, S. J., Kramer, A. F., Erickson, K. I., Scalf, P., McAuley, E., Cohen, N. J., Webb, A., Jerome, G. J., Marquez, D. X., & Elavsky, S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3316–3321. https://doi.org/10.1073/pnas.0400266101
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., Kim, J. S., Heo, S., Alves, H., White, S. M., Wojcicki, T. R., Mailey, E., Vieira, V. J., Martin, S. A., Pence, B. D., Woods, J. A., McAuley, E., & Kramer, A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. https://doi.org/10.1073/pnas.1015950108
Ferri, E., Marzetti, E., Calvani, R., Picca, A., Cesari, M., & Arosio, B. (2020). Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. International journal of molecular sciences, 21(15), 5236. https://doi.org/10.3390/ijms21155236
Fletcher, R.H., Fletcher, S.W., & Fletcher, G.S. (2014). Clinical Epidemiology: The Essentials. Fifth Ed. Lippincott Williams & Wilkins.
Gholamnejad, Z., Boskabady, M. H., & Jahangiri, Z. (2020). Exercise and Dementia. Advances in experimental medicine and biology, 1228, 303–315. https://doi.org/10.1007/978-981-15-1792-1_20
Irwig L, Irwig J, Trevena L, et al. Smart Health Choices: Making Sense of Health Advice. London: Hammersmith Press; 2008. Chapter 18, Relative risk, relative and absolute risk reduction, number needed to treat and confidence intervals. Available from: https://www.ncbi.nlm.nih.gov/books/NBK63647/
Mandsager, K., Harb, S., Cremer, P., Phelan, D., Nissen, S. E., & Jaber, W. (2018). Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA network open, 1(6), e183605. https://doi.org/10.1001/jamanetworkopen.2018.3605
Marks, B. L., Katz, L. M., Styner, M., & Smith, J. K. (2011). Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British journal of sports medicine, 45(15), 1208–1215. https://doi.org/10.1136/bjsm.2009.068114
Meng, Q., Lin, M. S., & Tzeng, I. S. (2020). Relationship Between Exercise and Alzheimer's Disease: A Narrative Literature Review. Frontiers in neuroscience, 14, 131. https://doi.org/10.3389/fnins.2020.00131
Murman D. L. (2015). The Impact of Age on Cognition. Seminars in hearing, 36(3), 111–121. https://doi.org/10.1055/s-0035-1555115
Nelson, M. E., Rejeski, W. J., Blair, S. N., Duncan, P. W., Judge, J. O., King, A. C., Macera, C. A., & Castaneda-Sceppa, C. (2007). Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and science in sports and exercise, 39(8), 1435–1445. https://doi.org/10.1249/mss.0b013e3180616aa2
Oudbier, S. J., Goh, J., Looijaard, S. M. L. M., Reijnierse, E. M., Meskers, C. G. M., & Maier, A. B. (2022). Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. The journals of gerontology. Series A, Biological sciences and medical sciences, 77(10), 1959–1968. https://doi.org/10.1093/gerona/glac121
Pedersen, B. K., & Febbraio, M. A. (2008). Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological reviews, 88(4), 1379–1406. https://doi.org/10.1152/physrev.90100.2007
Peterson, C. M., Johannsen, D. L., & Ravussin, E. (2012). Skeletal muscle mitochondria and aging: a review. Journal of aging research, 2012, 194821. https://doi.org/10.1155/2012/194821
Picca, A., Calvani, R., Bossola, M., Allocca, E., Menghi, A., Pesce, V., Lezza, A. M. S., Bernabei, R., Landi, F., & Marzetti, E. (2018). Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia. Biological chemistry, 399(5), 421–436. https://doi.org/10.1515/hsz-2017-0331
Rolland, Y., Pillard, F., Klapouszczak, A., Reynish, E., Thomas, D., Andrieu, S., Rivière, D., & Vellas, B. (2007). Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. Journal of the American Geriatrics Society, 55(2), 158–165. https://doi.org/10.1111/j.1532-5415.2007.01035.x
Severinsen, M. C. K., & Pedersen, B. K. (2020). Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocrine reviews, 41(4), 594–609. https://doi.org/10.1210/endrev/bnaa016
Stranahan, A. M., Lee, K., Becker, K. G., Zhang, Y., Maudsley, S., Martin, B., Cutler, R. G., & Mattson, M. P. (2010). Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiology of aging, 31(11), 1937–1949. https://doi.org/10.1016/j.neurobiolaging.2008.10.016
Smith, P. J., Blumenthal, J. A., Hoffman, B. M., Cooper, H., Strauman, T. A., Welsh-Bohmer, K., Browndyke, J. N., & Sherwood, A. (2010). Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine, 72(3), 239–252. https://doi.org/10.1097/PSY.0b013e3181d14633
Ströhle, A., Schmidt, D. K., Schultz, F., Fricke, N., Staden, T., Hellweg, R., Priller, J., Rapp, M. A., & Rieckmann, N. (2015). Drug and Exercise Treatment of Alzheimer Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Effects on Cognition in Randomized Controlled Trials. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry, 23(12), 1234–1249. https://doi.org/10.1016/j.jagp.2015.07.007
Szumilas M. (2010). Explaining odds ratios. Journal of the Canadian Academy of Child and Adolescent Psychiatry = Journal de l'Académie canadienne de psychiatrie de l'enfant et de l'adolescent, 19(3), 227–229.
Wang, S., Liu, H. Y., Cheng, Y. C., & Su, C. H. (2021). Exercise Dosage in Reducing the Risk of Dementia Development: Mode, Duration, and Intensity-A Narrative Review. International journal of environmental research and public health, 18(24), 13331. https://doi.org/10.3390/ijerph182413331
World Health Organization. (2022, September 20). Dementia. World Health Organization.
Retrieved December 2022, from https://www.who.int/news-room/fact-sheets/detail/dementia
Wu, H., Jang, J., Dridi, S., Ferrando, A. A., Wolfe, R. R., Kim, I. Y., & Baum, J. I. (2020). Net protein balance correlates with expression of autophagy, mitochondrial biogenesis, and fat metabolism-related genes in skeletal muscle from older adults. Physiological reports, 8(19), e14575. https://doi.org/10.14814/phy2.14575