Episode 141: Psychopharmacology Mediators
By listening to this episode, you can earn 1.5 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Maxwell Schauermann, MD, David Puder, MD
David Puder, M.D. has no conflicts of interest to report.
Joseph Goldberg, M.D. has the following conflicts of interest:
Consultant: BioXcel, Jazz Pharmaceuticals, Lundbeck, Otsuka, Sage Pharmaceuticals, Sunovion
Speakers bureau: Abbvie, Alkermes, Intracellular Therapies, Sunovion
Maxwell Schauermann, M.D., has no conflicts of interest to report.
Mediators of Treatment Response
“Mediators are factors that influence how well a treatment can work after it has commenced. They reflect the kinds of events in real-world settings that could derail or contaminate an otherwise efficacious mode of therapy”.
In this episode, we reviewed some of the mediators of treatment response that Dr. Goldberg outlines in chapter 5 of his new book, Practical Psychopharmacology: Translating Findings From Evidence-Based Trials into Real-World Clinical Practice. Some of the interesting points that I took away from reading his chapter on mediators are included below. It is notable that Dr. Goldberg mentions that some moderators (e.g., characteristics of the patient that influence treatment outcomes) can also be mediators of treatment response. Here we will be focusing on mediators, referring to events that occur to the patient after treatment has begun and influence treatment outcomes.
As an example of how a moderator can also be a mediator, Dr. Goldberg explains in his book that “executive dysfunction can itself moderate poor treatment response in major depressive disorder, while in older adult depression, poor executive function (notably, set shifting and semantic fluency) has been shown to mediate treatment noncompletion, but not nonremission during open treatment with venlafaxine” (Cristancho et al., 2018). As a reminder, set shifting is the ability to move back and forward between different tasks or mental sets and is often used as a measure of cognitive flexibility. Semantic fluency can be defined as a measure of executive functioning ability by one’s ability to name as many different fruits or animals under time pressure.
Treatment Adherence
Treatment adherence is perhaps the most notorious and regularly encountered mediator that we encounter as clinicians. Medication nonadherence is not a unique challenge for doctors, but in psychiatry we face additional barriers such as stigma towards psychotropics, patients with delusional beliefs or low motivation from their depression, anosognosia due to frontal lobe disruption that prevents them from recognizing the need for treatment, and medications with particular troublesome side effect burdens including sexual dysfunction, birth defects, and potential for serum toxicity that can cause organ damage. Psychiatric patients have nonadherence rates of 20-60% (Kreyenbuhl et al., 2016). Per NAMI, early studies of anosognosia have shown that about 30% of patients with schizophrenia and 20% of those with bipolar disorder have severe lack of insight into their diagnosis. Nonadherence is correlated with poorer outcomes including higher rates of hospital admission, suicide and overall mortality. How is one to convince a patient that they need lithium or depakote for their bipolar disorder when their illness prevents them from recognizing the need for treatment and the medications cause such concerning side effects?
Inquiring about nonadherence in a nonjudgmental fashion through normalizing the patient’s experience is essential to maintaining the therapeutic alliance. Physicians should attempt to problem solve nonadherence barriers and tailoring treatment regimens by considering, for example, fluoxetine for its extended half-life (parent drug has 4-6 days then an active metabolite for another 9.3 days, per Epocrates) in a patient with nonadherence. Explore with your patients if there are any issues they are experiencing that contribute to their nonadherence such as medication side effects, difficulty remembering to take the pills that may require behavioral modification interventions, or perhaps feeling that the medications are not doing anything for their symptoms. As Dr. Puder mentioned during the episode, you may also consider scheduling frequent visits during the first few visits with a patient to ensure their response to starting new medications and monitoring for their specific nonadherence barriers. Similar to how asking about suicidality decreases rates of completion, asking about nonadherence can only help improve treatment outcomes. Remember when you see your patients that the best predictor of future nonadherence is a history of past nonadherence. It is also important to note that rates of nonadherence differ across diagnoses and as Dr. Goldberg mentions in this podcast, even side effects themselves may differ with respect to diagnoses.
Dr. Goldberg outlines many different factors that are correlated with higher rates of medication noncompliance in his book, including demographic factors such as:
younger age
female sex
unemployment
higher levels of education
Some clinical factors that may also contribute include:
having adverse side effects from medications with the perception that it is unmanageable
cognitive dysfunction
severity of depressive symptoms
psychosis
having other psychiatric comorbidities
substance abuse
lack of social support
poor insight into the need for treatment
negative attitudes towards medications
a history of suicide attempts
early age of illness onset
shorter duration of illness
As mentioned in the podcast episode, it appears that patients who are less ill and also more severely ill would predictably have the worst medication adherence rates.
While there is always the potential for involuntary psychotropic treatment while hospitalized, it is always preferable to ally with the patient to find a treatment regimen that stabilizes their condition with the least amount of side effect burden.
Some ways to increase medication adherence include:
reducing dosing frequency as much as possible
clear and open communication about experiencing side effects
behavioral strategies such as placing medications next to one’s toothbrush as a cue to pair behaviors together
technological aids such as medication trackers or alarms to aid the patient in remembering to take their medications
Interestingly, a review of behavioral strategies for medication adherence suggests that motivational interviewing and other psychoeducational approaches are less effective for preventing nonadherence than those using behavioral tailoring (Kreyenbuhl et al., 2016).
Early Placebo Response
It is also important to remember that significant early response to treatment (<2 weeks) may indicate placebo response or that may not be a durable improvement. Therefore, do not assume that the patient has failed treatment if the significant improvement fades after 2 weeks, because this may simply represent the loss of placebo effect rather than a tachyphylaxis reaction to the medication.
Treatment Response At 2 Weeks
However, if there is a measurable improvement of 20% in the first 2 weeks, it can be a good indicator of future success for medications in a patient with major depressive disorder, bipolar depression, schizophrenia, panic disorder and generalized anxiety disorder. This measure comes from a study done in 2009 by Szegedi et al. The study concluded that “the high negative predictive values indicate little chance of stable response or stable remission in the absence of improvement within 2 weeks. A lack of improvement during the first 2 weeks of therapy may indicate that changes in depression management should be considered earlier than conventionally thought” (Szegedi et al., 2009). Thus, if the patient is showing no improvement after 2 weeks, following up a lack of change with things like a dosage change, augmentation, or substitutions is indicated.
Treatment Response At 6 Weeks
For certain medications, it is important to remind your patients to wait until at least 6 weeks into treatment before deciding if the pharmacotherapy choice is helping. In Dr. Goldberg’s book, he writes that “antidepressant response or remission achieved by week 6 may be one of the strongest predictors (mediators) of 12-month remission status from depression” (Ciudad et al., 2012, p.102).
Drug-drug interactions
Drug-drug interactions are something all astute clinicians consider when formulating treatment plans. Many of the pharmacotherapies used in psychiatry have interactions with each other. Cleverly, psychiatrists have harnessed some of these combinations to produce synergistic effects such as fluvoxamine increasing the blood level of clozapine or fluoxetine increasing the dose of risperdal.
Dr. Goldbergs outlines some these important examples in his book, including:
Patients stably taking a psychotropic drug that is highly bound (>85%) to plasma proteins (e.g., carbamazepine, divalproex, diazepam, prazosin) who then begin taking another drug that competes for an displaces protein binding (e.g., warfarin, aspirin, naproxen, ibuprofen, indomethacin, furosemide, etc.) resulting in increased free drug plasma concentrations with potential for toxicity or overactivity. If protein binding displacement is of potential concern, one could consider measuring serum unbound fractions of the drug to obtain a more meaningful estimate of its pharmacokinetic and possibly pharmacodynamic bioavailability.
Oral contraceptives can interfere with oxidation and clearance of benzodiazepines such as alprazolam, chlordiazepoxide, and diazepam. They may also effectively inhibit the clearance of beta-blockers, tricyclic antidepressants, and corticosteroids.
Adding an NSAID to Li can potentially increase serum Li levels by ~20% causing potentially life threatening toxicity.
Patients who quit smoking (during in inpatient psychiatric hospitalization), begin taking clozapine, stabilize on it, and then start smoking again in their home environment can often have a psychotic relapse due to hydrocarbons from smoking, causing induction of clozapine via cytochrome P450 1A2 metabolism, thus decreasing the effectiveness of clozapine.
Dr. Goldberg summarizes conveniently a list of common CYP450 inducers and inhibitors in his book on page 100:
Inducers of CYP450:
1A2: broccoli, brussel sprouts, chamomile tea, charred meat, omeprazole
3A4: prednisone, primidone, St. John’s Wort
2C9: phenytoin
2D6: dexamethasone
Multiple:
carbamazepine (1A2, 2C9, 2C19, 2D6)
modafinil (1A2, 3A4/5)
phenobarbital (1A2, 2C9, 2D6, 3A4)
rifampin (2C9, 2C19)
Inhibitors of CYP450:
1A2: ciprofloxacin
3A4: corticosteroids, cyclosporine, diltiazem, grapefruit juice, protease inhibitors, verapamil
2C19: omeprazole
Multiple:
amiodarone (2C9, 3A4)
clarithromycin, erythromycin (1A2, 3A4)
fluconazole (2C9, 2C19, 3A4)
Dr. Goldberg also provided a helpful “fire analogy” during this episode that emphasizes the importance of achieving treatment remission as soon as possible after beginning treatment. The analogy goes as follows:
Acute intervention phase = The fire department arrives to extinguish the house on fire. The firemen assess whether the fire is responding to their efforts. If treatment is responding by >50% then we consider the patient entering remission.
Continuation phase = The firemen sweep the area for any embers that might rekindle the flames. Similarly, doctors assess closely during the first 4-6 months to monitor for relapse of symptoms. While symptoms are only recently remitting, there is risk that the patient may have relapse of symptoms during this period.
Recovery phase = The fire is now out after 4-6 months of fighting the fire, so if another fire begins after this point, then we would consider it a new fire. As patients get further and further from their last episode, they become more stable and less likely to have a relapse of symptoms.
New Stressors
Interpersonal & psychosocial life disruptions that occur after treatment has started are major mediators that can increase symptoms despite a medication that might have been working. Patients may start a new psychotropic medication only to have its effectiveness negatively mediated by experiencing a tragedy in their life or receiving awful news of some sort, or the patient may have to tolerate living at home in a toxic environment.
Expressed emotion
One clinical predictor of symptom relapse that I found interesting as I read through the chapter is that of expressed emotion (EE), which is used in clinical studies as a relapse factor in schizophrenia, major depression, and bipolar studies refers to “the critical, hostile, or emotionally over involved communication styles within families that can undercut otherwise favorable moderators and mediators of successful treatment outcomes (such as therapeutic drug doses and levels, or appropriate medication adherence).” Research by George Brown and colleagues in the 1950s observed 229 men, of which 156 were schizophrenic, after being recently discharged from a psychiatric hospital and surprisingly found that those who stayed with their parents or wives had the highest rates of relapse when compared to those who lived in “lodgings” or with siblings. Additionally, to stay with either their parents/wives who experienced the critical, hostile, or emotionally over-involved behavioral patterns could lead to worsening stimulation and social withdrawal.
Examining the communication styles between a patient and their family is imperative to determine if expressed emotion is a significant contributing negative mediator. This clinical predictor underlines the importance of educating families about mental health conditions and supporting families to decrease rates of caregiver burnout so they can be allies instead of deterrents to our patient’s wellness. A clinically useful way to formally evaluate EE is with the Level of Expressed Emotion (LEE) scale to get a predictive value for relapse across various diagnoses after completing a 60-item questionnaire that typically takes 10-15 minutes. Higher scores correlate with a higher likelihood of symptom relapse.
Cognitive functioning
Changes in cognitive functioning is yet another mediator of treatment response. Many psychiatric conditions can affect one’s cognition, but when cognition is negatively impaired by depression there is an interesting concept of “hot and cold cognition” that Tranter et al., 2009 observed to predict whether a patient will have early treatment response. Specifically, Dr. Tranter found that if, after 2 weeks, the patients could better recognize happiness, then at 6 weeks they were more likely to have less symptoms (Pearson correlation = 0.46).
Similarly, in a retrospective study by Gorwood et al. in 2015, patients with an increase in joy after two weeks of treatment with agomelatine had a greater predictive value for eventual treatment response with an antidepressant or remission of symptoms when compared with a reduction in sadness over the same period.
There are many moderating factors that affect treatment outcomes in regards to cognition, such as a history of TBI, chronic lyme disease, cerebrovascular accidents, history of stroke and poststroke depression, and extensive substance abuse. Cognitive impairment also presents differently depending on the diagnosis that you are treating. For example, Dr. Goldberg mentions in the podcast that verbal memory, attentional processing, and executive functioning are the three primary domains that are affected in bipolar disorder. In schizophrenia, however, global cognitive functioning is impaired and is less circumscribed to individual domains. Working memory, which allows one to hold small amounts of information and apply it to solve problems in real time, is markedly impaired when compared to bipolar disorder.
When treating such cognitive impairments, we would expect improvement of symptoms after starting a cognitive enhancing treatment. Intuitively, lack of improvement in symptoms portends a worse prognosis. When examining the CATIE study, Dr. Goldberg notes that second generation antipsychotics were not shown to improve cognitive functioning.
Some off-label treatment options for negative symptoms include the following:
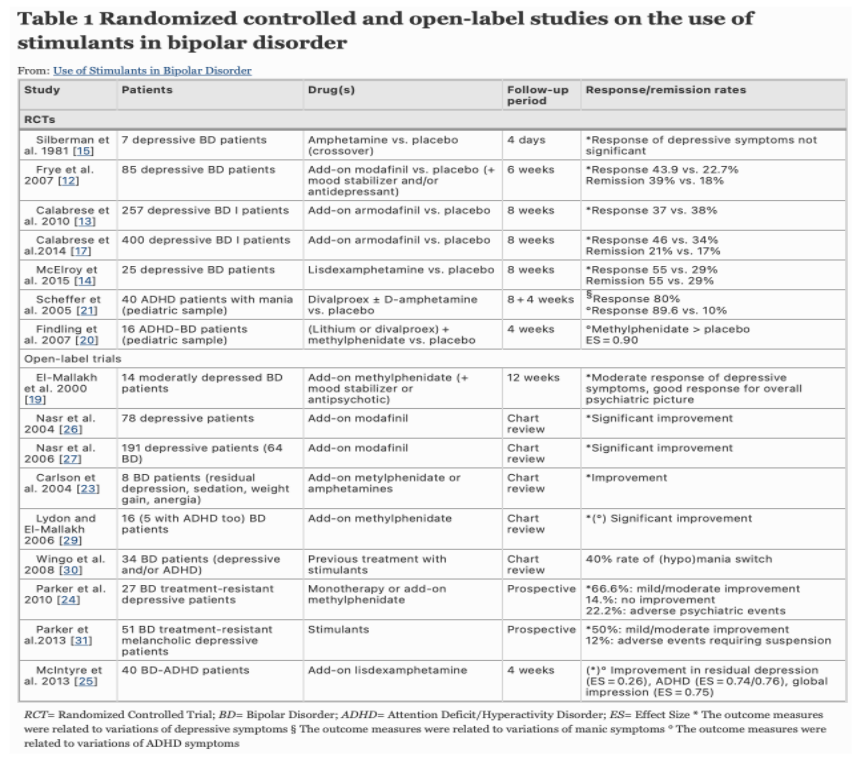
Stimulants such as methylphenidate and modafinil which improves associative fluency in bipolar depression
Remember that when treating bipolar patients with psychoactive stimulants or SSRI, highly consider concurrently treating with a mood stabilizer so as not to precipitate a manic episode. There is not enough evidence at this time to support safe usage of stimulants in bipolar patients without a concurrent mood stabilizer. Should manic symptoms present after starting a stimulant, then discontinuation of the stimulant is appropriate. Other concerns that may arise from this off-label treatment option include misuse of stimulants, mixed states, and rapid cycling (Perugi et al., 2017).
Dopamine agonists such as bupropion or pramipexole. Pramipexole specifically helps with lower levels of motivation, anergia, and impaired cognitive processing in patients with major depression and bipolar depressed patients. In euthymic bipolar patients, pramipexole helps more with social cognition and social functioning (Blumberg et al., 2020).
Dopamine antagonists such as lurasidone which also antagonizes 5HT7 to achieve improvement in bipolar global cognitive functioning. Lurasidone at minimum dose of 120 mg was also shown to improve cognitive function over seroquel in schizophrenic patients (Franklin et al., 2015).
Pro-cholinergics such as donepezil which have off-label data for improving memory dysfunction in TBI patients, cognitive function in vascular dementia and other forms of dementia. Donepezil has not been shown to help with cognitive impairment from depression or bipolar (Kumar et al., 2021).
Vortioxetine has been shown to have good treatment outcomes for cognitive dysfunction apart from improving cognition simply by treating depression in MDD (Mahableshwarkar, Atul R et al., 2016). Cognitive domains improved include speed of processing, verbal learning, and memory.
Olanzapine + Fluoxetine (OFC) is used as a treatment-resistant treatment option for bipolar depression. Studies have shown it to produce markedly increased levels of dopamine and serotonin in the prefrontal cortex, suggesting that it would improve cognitive impairments in those with severe illness. However, Dr. Goldberg cautions that this expectation may produce an expectancy bias that does not actually pan out in the clinical world. In my review of the literature on PubMed, there are currently no studies evaluating OFC’s effect on cognition.
Motivational interviewing (MI) has become a buzzword in the world of addiction and serves as an evidence-based collaborative method for enhancing a patient’s motivation for treatment of their substance use. Very often, our patients use substances to self-medicate their own symptoms of anxiety, insomnia, and depression. It is important to recognize the utility of substances as a coping skill in our patients’ lives in order to foster empathy and the therapeutic relationship. MI allows a clinician to role with the resistance that a patient may have towards cessation of usage or enrolling in treatment. The steps and qualities of MI are reviewed below:
The clinician should take a stance of guiding the patient in their decision making, but one must be careful not to be overly dogmatic in giving advice and instead actively listen to the patient’s desires. One should view the patient as an equal and refrain from confrontations or unsolicited advice.
MI enables patients to make changes by allowing them to create their own meaning and importance for change. MI takes a stance of respect and curiosity towards the patient to better understand their unique challenges and honor their autonomy to change.
The emphasis of MI is on how a patient experiences the relationship with their clinician. The focus should be on creating a partnership with the patient, empowering them to align their values with their behaviors, being nonjudgmental and compassionate. The primary skills that a clinician should utilize are nicely summarized by the mnemonic “OARS”:
- Open ended questions to explore their experience
- Affirmation of their efforts
- Reflecting to the patient what you have heard to demonstrate active listening
- Summarizing to emphasize key points
Now that we have an understanding of what MI is, when should we use MI in our encounters? MI is a board tool that can be used in many situations, but it is best employed for situations where a patient is highly ambivalent about making a change, has low levels of confidence in their ability to change, low desire to change, and/or low insight into the severity of their illness or the benefits of changing their behavior.
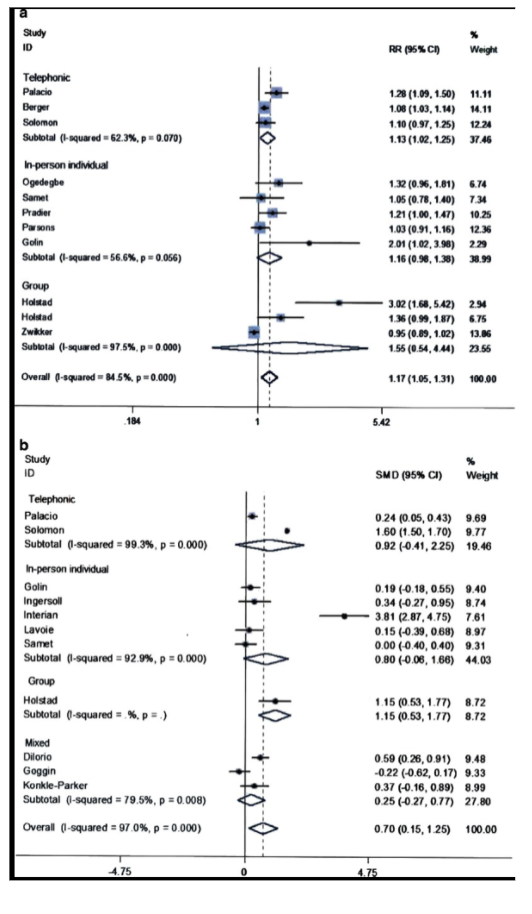
In a meta-analysis of randomized-controlled trials (RCTs) examining if MI improved medication adherence in patients receiving HAART, investigators looked at the MEDLINE database of RCTs comparing MI to a control group with reported measures of medication adherence from 1966 to February, 2015. Results showed that out of 11 RCTs, the pooled relative risk (RR) was 1.17 (95% CI, CL 1.05 - 1.31, p< 0.01). Characteristics of MI that were significantly associated (p < 0.05) with medication adherence included telephonic MI and “fidelity based feedback”, group MI, and “fidelity assessments among studies reporting continuous measures and delivery” by staff.
(Palacio A et al., 2016).
Summary:
Thinking about mediators in follow up visits can better help us understand how to achieve our patients’ goals.
Works Cited
“Anosognosia.” NAMI, https://www.nami.org/about-mental-illness/
common-with-mental-illness/anosognosia.
Blumberg MJ, Vaccarino SR, McInerney SJ. Procognitive Effects of Antidepressants
and Other Therapeutic Agents in Major Depressive Disorder: A Systematic
Review. J Clin Psychiatry. 2020 Jul 21;81(4):19r13200. doi:
10.4088/JCP.19r13200. PMID: 32726521.
Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry.
2013 Sep;26(5):446-52. doi: 10.1097/YCO.0b013e3283642da4. PMID: 23880592;
PMCID: PMC4222796.
Franklin, Rachel et al. “Lurasidone for the treatment of bipolar depression: an
evidence-based review.” Neuropsychiatric disease and treatment vol. 11 2143-52.
19 Aug. 2015, doi:10.2147/NDT.S50961
Hsu WY, Lane HY, Lin CH. Medications Used for Cognitive Enhancement in Patients
With Schizophrenia, Bipolar Disorder, Alzheimer's Disease, and Parkinson's
Disease. Front Psychiatry. 2018 Apr 4;9:91. doi: 10.3389/fpsyt.2018.00091.
PMID: 29670547; PMCID: PMC5893641.
Kreyenbuhl J, Record EJ, Palmer-Bacon J. A review of behavioral tailoring strategies
for improving medication adherence in serious mental illness. Dialogues Clin
Neurosci. 2016 Jun;18(2):191-201. doi: 10.31887/DCNS.2016.18.2/jkreyenbuhl.
PMID: 27489459; PMCID: PMC4969706.
Kumar, Anil et al. “Donepezil” StatPearls. 7 May 2021. PubMed ID: 30020629. 8/12/21.
Mahableshwarkar, Atul R et al. “A Randomized, Placebo-Controlled, Active-Reference,
Double-Blind, Flexible-Dose Study of the Efficacy of Vortioxetine on Cognitive
Function in Major Depressive Disorder.” Neuropsychopharmacology : official
publication of the American College of Neuropsychopharmacology vol. 40,8
(2015): 2025-37. doi:10.1038/npp.2015.52
Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational Interviewing
Improves Medication Adherence: a Systematic Review and Meta-analysis. J Gen
Intern Med. 2016 Aug;31(8):929-40. doi: 10.1007/s11606-016-3685-3. Epub
2016 May 9. PMID: 27160414; PMCID: PMC4945560.
Perugi, G., Vannucchi, G., Bedani, F. et al. Use of Stimulants in Bipolar Disorder. Curr
Psychiatry Rep 19, 7 (2017).
https://0-doi-org.catalog.llu.edu/10.1007/s11920-017-0758-x
“Understanding Motivational Interviewing.” Understanding Motivational Interviewing |
Motivational Interviewing Network of Trainers (MINT), https://motivationalinterviewing.org/understanding-motivational-interviewing.
Zhang, W., Perry, K., Wong, D. et al. Synergistic Effects of Olanzapine and Other
Antipsychotic Agents in Combination with Fluoxetine on Norepinephrine and
Dopamine Release in Rat Prefrontal Cortex. Neuropsychopharmacol 23,
250–262 (2000). https://doi.org/10.1016/S0893-133X(00)00119-6