Episode 174: “Serotonin Toxicity”, Otherwise known as Serotonin Syndrome
By listening to this episode, you can earn 1.25 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Alexander Horwitz, MD, Ken Gillman, MD, David Puder, MD
Conflicts of interest for this episode: Dr. Gillman has equity interests in and is on the advisory board of NeuraWell Therapeutics, the company that has the patent for a modified form of tranylcypromine.
Introduction
In today’s episode of the podcast, we are joined by psychiatrist and neuropharmacologist Dr. Ken Gillman who is the founder and convener of the International MAOI (monoamine oxidase inhibitor) Expert Group and widely recognized as a world expert in serotonin toxicity.
Serotonin toxicity (syndrome) is a rare as well as potentially lethal form of toxicity that results from excess serotonin within neuronal synapses. There are numerous poorly written/controlled case reports that have perpetuated misinformation about drugs that can cause serotonin toxicity. While the word “syndrome” is often used, toxicity is a more accurate description given that toxicity represents a spectrum of severity rather than a defined set of symptoms. In today’s podcast, we will discuss the pathophysiology, causes, clinical presentation, criteria, controversies, and medical management of serotonin toxicity.
Definition and Clinical Presentation
Serotonin toxicity is a direct result of ingesting drugs that substantially increase brain concentrations of serotonin. It can be thought of as a drug-induced toxicity caused by serotonin poisoning. Toxicity is viewed as a more accurate description than syndrome for a variety of reasons. In medicine, the term syndrome is a broad definition often used to encompass a set of signs or symptoms without necessarily attributing them to a single identifiable pathogenesis. Syndrome is also often used to imply that something is idiosyncratic and occurs only in certain people and not others, such as neuroleptic malignant syndrome (NMS). Toxicity implies a spectrum as opposed to a circumscribed set of symptoms. Lithium overdose is not referred to as lithium syndrome – why should this be the case with serotonin?
Excess serotonin produces a spectrum of severity with clinical effects ranging from mild signs and symptoms (which may be underdiagnosed) such as tremor and diaphoresis, all the way to more serious effects including muscular hypertonicity and hyperthermia. It is useful to characterize serotonin toxicity as a clinical triad:
Neuromuscular abnormalities (particularly hyperactivity): hyperreflexia, hyperkinesia, tremor, clonus, and pyramidal rigidity (in severe cases)
Autonomic hyperactivity: mydriasis, hyperactive bowel sounds, tachycardia, tachypnea, hyperthermia, diaphoresis
Altered mental status: agitation and hyperarousal.
Symptoms of serotonin toxicity develop rapidly (over hours as opposed to days). Neuromuscular signs are often more observable in the lower limbs. While there are multiple non-specific symptoms associated with serotonin toxicity (e.g., diaphoresis and tachycardia), as discussed below, clonus is the single most important sign for diagnosing serotonin toxicity. Clonus often starts in the lower limbs and then becomes more generalized.
Pathophysiology
Serotonin toxicity is mediated through 5-HT2A [5-HT = 5-hydroxytryptamine = serotonin] receptor agonism. It was initially thought that 5-HT1A agonism contributes to serotonin toxicity, but it was later shown that 5-HT1A antagonists have a negligible effect on the treatment of serotonin toxicity and that 5-HT1A agonists cause hypothermia (not hyperthermia). In order to induce serotonin toxicity, a drug must increase serotonin concentrations by 50-100 times above baseline. Severe toxicity will result in intrasynaptic serotonin concentrations 1000 times above baseline.
Dr. Gillman has noted three classes of drugs that, in certain combinations at therapeutic doses, can lead to severe serotonin toxicity:
Monoamine oxidase inhibitors (MAOIs)
Serotonin reuptake inhibitors [including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs); given that the word “selective” in SSRI is largely a misnomer, moving forward, they will be referred to [S]SRIs]
Serotonin releasers [e.g. MDMA (“ecstasy”)]
While there are other combinations of drugs/medications that can possibly or rarely cause serotonin toxicity, with normal therapeutic doses, only [S]SRI plus MAOI is likely to lead to severe serotonin toxicity. The risk of an MAOI precipitating serotonin toxicity is related to its inhibition of monoamine oxidase A (MAO-A), which is responsible for the breakdown of serotonin in addition to norepinephrine and dopamine. Monoamine oxidase B (MAOI-B) does not metabolize serotonin.
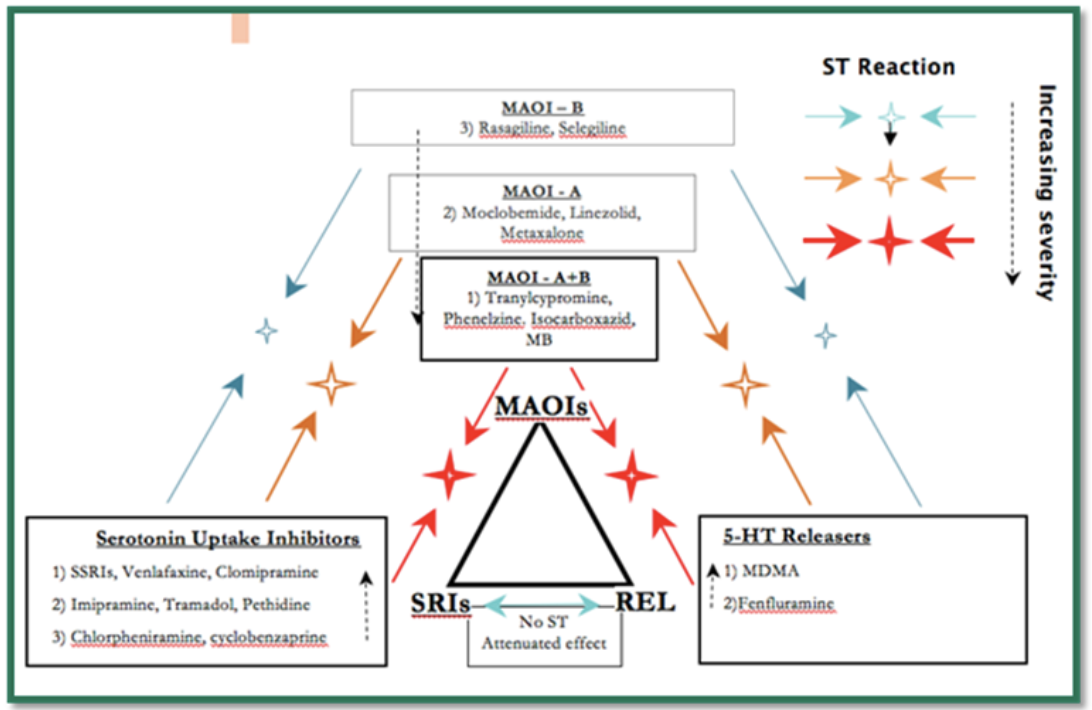
The following diagram was taken from Dr. Gillman’s website, psychotropical.com, and is a schematic way of considering interactions between the three classes of drugs that can lead to severe serotonin toxicity:
Of note, [S]SRIs block the entry of MDMA into the serotonergic neuron via SERT and therefore prevent the release of excess serotonin and serotonin toxicity.
Overdose with a single [S]SRI leads to moderate serotonin toxicity (requiring inpatient admission and medical treatment) in only 10-20% of cases, but with no fatalities or hyperthermia [i.e., temperature over 38.5 degrees Celsius or 101.3 degrees Fahrenheit]. Combining an MAOI with a [S]SRI leads to severe serotonin toxicity in about 50% of cases (even with therapeutic doses).
Criteria
There are two major criteria for the diagnosis of serotonin toxicity: the Sternbach Criteria and the Hunter Criteria. The Sternbach Criteria were proposed by psychiatrist Dr. Harvey Sternbach in 1991. Dr. Sternbach reviewed a total of 38 cases from 10 case reports and two case series published in the literature to identify the most common signs and symptoms. In 2003, Professor Ian Whyte (a medical toxicologist) and colleagues published new criteria for serotonin toxicity.
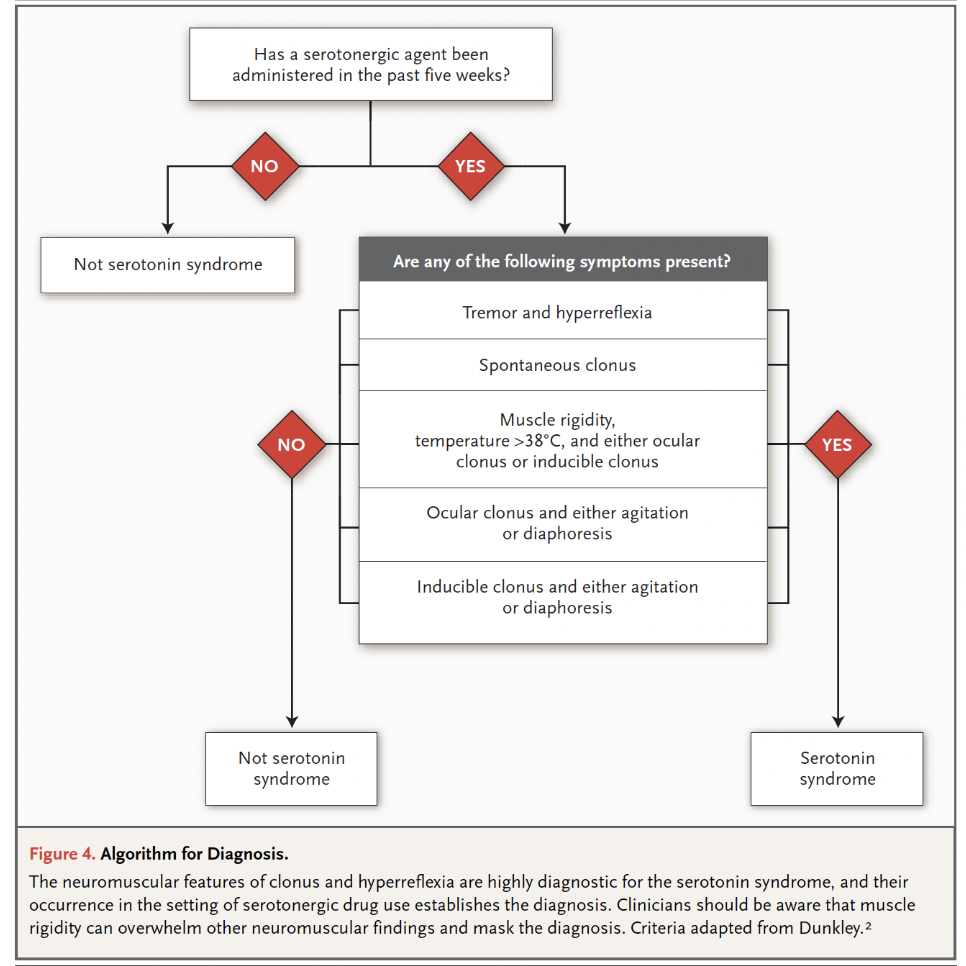
The Hunter Serotonin Toxicity Criteria published by Whyte and colleagues examined all patients admitted to the Hunter Area Toxicology Service (HATS) located in the Hunter Area of New South Wales, Australia, from January 1987 to February 2003 (n = 9960). Data collection was performed by a team of medical toxicologists, which yielded prospective data that was much more reliable than Sternbach’s 38 case reports. The 2003 paper identified 2222 cases of serotonin toxicity (thousands more cases have been subsequently added to the HATS database) and noted that only the following variables were required for accurately predicting serotonin toxicity: spontaneous clonus, inducible clonus, ocular clonus, agitation, diaphoresis, tremor, and hyperreflexia. It is important to note that serotonin toxicity represents a spectrum of symptoms that progress as follows: tremor 🡪 hyperreflexia 🡪 inducible clonus 🡪 spontaneous clonus 🡪 muscular rigidity.
The Hunter Criteria as originally published:
Whyte and colleagues found the new criteria to be simpler, in addition to being more sensitive (84% versus 75 %) and specific (97% versus 96%), compared to the Sternbach Criteria. While the research from the Hunter group provided more sensitive and specific criteria than the Sternbach Criteria, they are research diagnostic criteria and should not be overly relied on when making a clinical diagnosis.
Here is an easier way of looking at the Hunter Criteria (Boyer et al 2005):
Specific Medications and Controversies
A variety of drugs, including non-psychotropic medications, have greater or lesser potency at serotonin inhibition and therefore might be associated with serotonin toxicity. In addition, there are some other medications that have incorrectly been linked to serotonin toxicity, which is largely due to an excess of poorly controlled case reports.
Some analgesics have weak serotonin reuptake properties and may be associated with serotonin toxicity. Tramadol, in addition to being a synthetic opioid, is also an inhibitor of SERT. The antibiotic linezolid acts as an MAOI. The antipsychotic ziprasidone weakly inhibits SERT, in addition to the norepinephrine transporter (NET), and can to serotonin toxicity when combined with MAOIs. Methylene blue has multiple medical uses but notably acts as a potent MAOI. When treating a patient with serotonin toxicity, it is imperative to consider all of their medications, not just their psychiatric medications. In addition, it is necessary to consider cytochrome P450 interactions.
Tricyclic Antidepressants (TCAs) and MAOIs:
It is safe to combine therapeutic doses of TCAs and MAOIs except in two cases: clomipramine and imipramine, which are both relatively potent inhibitors of SERT. The other TCAs do not have enough serotonin reuptake inhibition potency to cause serotonin toxicity when combined with MAOIs.
The following medications have been incorrectly associated with serotonin toxicity:
Mirtazapine:
Mirtazapine is commonly referred to as a noradrenergic and specific serotonin antidepressant (NaSSA), although the pro-serotonergic effect of mirtazapine is dubious at best. The idea that mirtazapine can lead to an increase in serotonin initially came from microdialysis studies in animals that have not been replicated. The pro-serotonergic effects of a medication in humans can be elucidated in a variety of ways: the clinical efficacy for a particular illness thought to have a serotonergic component [e.g., obsessive compulsive disorder (OCD) and cataplexy], serotonergic side effects (e.g., sexual dysfunction), toxicity in single drug overdose, and serious or fatal serotonin toxicity if mixed with MAOIs. Mirtazapine fails in each of these respects. Mirtazapine pretreatment has actually been shown to abolish hyperthermia in an animal model of serotonin syndrome.
Mirtazapine is ineffective for the treatment of both OCD and cataplexy, does not have sexual side effects, is unlikely to lead to major toxicity in overdose, and can safely be combined with MAOIs. The primary serotoninergic effect of mirtazapine is to antagonize 5-HT2A receptors; as noted above, agonism of 5-HT2A receptors leads to serotonin toxicity. In addition, mirtazapine has virtually no activity at the SERT.
Trazodone:
Trazodone is often referred to as a serotonin antagonist and reuptake inhibitor (SARI). Trazodone, like mirtazapine, is an antagonist of 5-HT2A receptors. Similar to [S]SRIs, trazodone also inhibits the SERT. Despite this, trazodone is not a significant inhibitor of the SERT and its antagonism of 5-HT2A receptors is much more robust.
Similar to mirtazapine, trazodone is ineffective for the treatment of both OCD and cataplexy, does not cause sexual side effects, is unlikely to lead to major toxicity in overdose, and can safely be combined with MAOIs.
Triptans:
In 2006, the FDA issued an alert about potentially life-threatening serotonin toxicity with triptans when combined with [S]SRIs and SNRIs (serotonin and norepinephrine reuptake inhibitors) based on 29 case reports. In 2009, in response to the FDA alert, Dr. Gillman noted that the affinity of triptans for the 5-HT2A receptor is thousands of times less than the affinity for the 5-HT1D receptor. He also noted that in rodents given 100 times the therapeutic dose of naratriptan, no behavioral effects relevant to serotonin toxicity were observed.
Dr. Gillman also criticized the quality of the case reports, which were often informal, “second-hand,” non-peer-reviewed, and did not use validated criteria to diagnose serotonin toxicity. Shortly after Dr. Gillman’s paper, and also in response to the FDA warning, the American Headache Society put out a position paper examining the 29 cases. Of the 29 cases, 10 met the Sternbach criteria while none met the more sensitive and specific Hunter Criteria.
The authors noted that triptans are agonists of 5-HT1B and 5-HT1D receptors and would likely not lead to serotonin toxicity, which requires stimulation of 5-HT2A receptors. The authors noted “currently available evidence does not support limiting the use of triptans with [S]SRIs or SNRIs, or the use of triptan monotherapy,” but warned “given the seriousness of serotonin syndrome, caution is certainly warranted, and clinicians should be vigilant to serotonin toxicity symptoms.”
Orlova et al. (2018) examined 14 years of electronic health records and identified 19,017 patients who were concurrently taking a triptan and an [S]SRI or SNRI. They identified 17 patients with “possible serotonin syndrome” and two patients who were classified as having a “definite serotonin syndrome.” The authors noted that most of the 17 patients were taking a large number of other medications, which could lead to conditions mimicking serotonin toxicity.
Buspirone:
Buspirone is a 5-HT1A partial agonist. Buspirone has been given with MAOIs and there is no compelling evidence that buspirone leads to serotonin toxicity. As noted previously, 5-HT1A agonism causes hypothermia (not hyperthermia). Like the other medications in this this list, case reports of buspirone causing serotonin toxicity have been reviewed by Dr. Gillman and found to be poorly controlled.
Ondansetron:
Since 2012, the FDA and World Health Organization (WHO) have released reports concluding that there is a potential risk for serotonin toxicity when ondansetron is combined with [S]SRIs. Ondansetron is an antagonist of 5-HT3 receptors and does not possess agonist properties or appreciably increase serotonin concentrations. Ondansetron was thoroughly studied for the treatment of fluvoxamine-induced nausea. Fluvoxamine is a potent inhibitor of SERT. The combination of fluvoxamine and ondansetron did not lead to any observed serotonergic side effects.
Psilocybin and LSD:
Both classic psychedelics are agonists of 5-HT2A receptors, but have not yet been clearly implicated in causing serotonin toxicity despite many multiple poorly controlled case reports purporting to show serotonin toxicity with the drugs. While psilocybin and LSD have not yet been shown to be clear precipitators of serotonin toxicity, caution is still warranted.
Dr. Gillman has hypothesized that although these drugs are potent agonists of 5-HT2A receptors, the concept of agonist-directed signaling might account for the fact that they do not seem to cause serotonin toxicity. Agonist-directed signaling refers to the fact that different agonists can trigger diverse signaling pathways while acting on the same receptor. The serotonin toxicity model is useful for elucidating pharmacologic mechanisms of action; Dr. Gillman, using his knowledge of serotonin toxicity, was able to hypothesize and prove that methylene blue was an MAOI. It is possible that serotonin toxicity will continue to elucidate drug mechanisms of action and to help us better understand functional selectivity.
Differential Diagnosis (Chalk and Cheese)
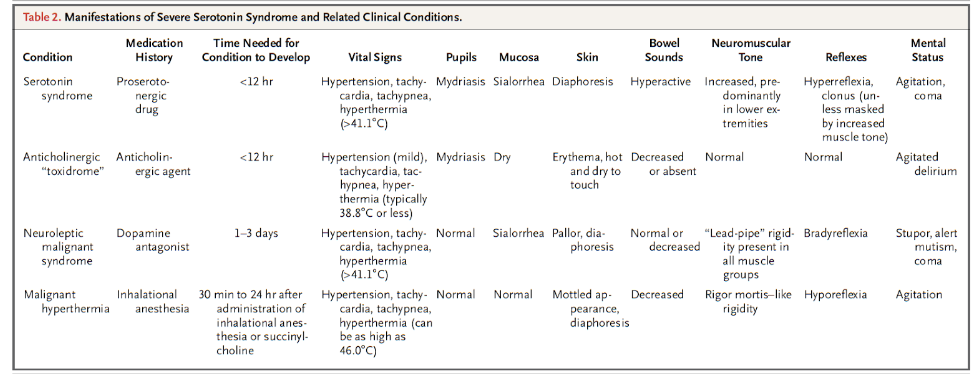
When considering the differential diagnosis of serotonin toxicity, NMS is inevitably brought up given the relative similarities: both are drug-induced, potentially life-threatening, and can lead to neuromuscular and autonomic abnormalities. Despite their similarities, the number of stark differences between the two led Dr. Gillman and Professor Whyte to note that they are as similar as “chalk and cheese.” Serotonin toxicity is caused by drugs that increase intrasynaptic concentrations leading to stimulation of 5-HT2A receptors while NMS is caused by dopamine antagonists.
As noted above, serotonin toxicity is a predictable response to serotonin poisoning and occurs on a spectrum, while NMS is idiosyncratic. Serotonin toxicity develops much more rapidly (i.e., hours) compared to NMS, which is much more insidious and develops over days. Serotonin toxicity leads to hyperkinesia while NMS leads to bradykinesia.
The following table was taken from Boyer et al. (2005) and is a useful summary for comparing severe serotonin toxicity to other clinical conditions:
Historical Side Note: Libby Zion
Modern medical resident work hours, including the maximum 80-hour work week averaged over four weeks and the 24-hour limit of continuous duty, are largely due to a case of serotonin toxicity that led to the death of a young patient.
In 1984, Libby Zion was an 18-year-old college student who presented to New York Hospital (Weill Cornell Medical Center) with an unidentified flu-like illness. Prior to her presentation, Zion was prescribed chlorpheniramine by her primary care physician for her illness. On admission, Zion was noted to have jerking movements. The admitting team was composed of an intern and second year resident who, after discussing the case with the supervising attending physician, prescribed meperidine (an opioid pain medication that is now more widely recognized as a serotonin reuptake inhibitor) to help with Zion’s jerking movements. Meperidine in combination with the MAOI, phenelzine, which Zion was already taking for depression, and chlorpheniramine, an antihistamine and SERT inhibitor, led to a severe case of serotonin toxicity. Zion’s temperature reached 107 degrees Fahrenheit and she had to be chemically and physically restrained due to agitation. She would eventually die from cardiac arrest.
At the time, the serotonergic effects of meperidine were not well-known. Zion’s parents believed that her death was primarily the result of understaffing at the hospital as well as extreme resident work hours. Of note, Libby was the daughter of Sidney Zion who was a lawyer and writer for The New York Times.
Libby’s parents' indignation about their daughter’s death led to a state investigation, civil trial, and the creation of the Bell Commission to investigate resident work hours. The state investigation ended with the Board of Regents voting to “censure and reprimand” both residents for acts of gross negligence, although they were able to continue practicing medicine. The civil trial led to both residents, along with Libby Zion’s primary care physician, having to pay $375,000 dollars in restitution to the Zion family for negligence. Eventually, the New York State Department of Health Code, Section 405 (also known as Libby Zion’s Law), was created, which mandated the 80-work week and 24-hour limit of continuous duty.
Medical Management
Pharmacologists and toxicologists now prefer to use as little medication as possible to treat a toxidrome caused by other drugs. The priority is to stabilize the patient with conservative management. Treatment depends on the type and quantity of drugs ingested and the evolution/rate of change of symptoms. Dr. Gillman notes that although supportive/non-specific treatment is sometimes all that is needed, not understanding the spectrum concept or failing to take complex risk factors into consideration is dangerous.
Benzodiazepines are often useful for agitation, reducing hyperkinesia, and have also been found to reduce temperature in rats with serotonin toxicity. For severe serotonin toxicity, such co-ingestion of a MAOI plus [S]SRI, toxicologists would recommend transferring the patient to an ICU. The following is possibly indicated with severe serotonin toxicity resulting from co-ingestion of a MAOI and [S]SRI: intubation, neuromuscular paralysis, and active cooling. 5-HT2A antagonists (cyproheptadine or chlorpromazine) are no longer routinely recommended or used.
Take-Home Points
Serotonin toxicity is a result of drugs that increase intrasynaptic concentrations of serotonin by at least 50-100 times above baseline.
Serotonin toxicity is a form of serotonin poisoning.
Serotonin toxicity is the preferred term by experts: toxicity is a more accurate description, given that toxicity represents a spectrum rather than a well-defined set of symptoms or idiosyncratic reaction (i.e., syndrome).
Serotonin toxicity is mediated through agonism of 5-HT2A receptors (not 5-HT1A receptors).
Serotonin toxicity consists of a clinic triad of neuromuscular hyperactivity, autonomic hyperactivity, and altered mental status.
The Hunter Criteria were based on over 2000 cases of serotonin toxicity; data collection was performed by a team of medical toxicologists, which yielded data that was much more reliable than Sternbach’s 38 case reports.
The Hunter Criteria are more sensitive and specific than the Sternbach Criteria.
HATS found that only the following variables were required for predicting serotonin toxicity: spontaneous clonus, inducible clonus, ocular clonus, agitation, diaphoresis, tremor, and hyperreflexia.
Clonus is the single most important sign for diagnosing serotonin toxicity.
There are many poorly written/controlled case reports which promulgate inaccuracies about drugs that cause serotonin toxicity.
Mirtazapine, trazodone, triptans, buspirone, and ondansetron do not cause substantially increase intrasynaptic concentrations of serotonin and therefore do not cause serotonin toxicity.
There are three classes of drugs that, in certain combinations at therapeutic doses, can lead to severe serotonin toxicity: MAOIs, [S]SRIs, and serotonin releasers.
Adding MDMA to an [S]SRI will not cause serotonin toxicity.
Some non-psychiatric medications not usually regarded as psychiatric act as relatively weak SRIs.
When taken alone in overdose, SRIs lead to hospitalization 10-20% of the time but do not result in serious complications including rigidity and hyperthermia greater than 38.5 degrees Celsius or 101.3 degrees Fahrenheit.
Combining an MAOI with an [S]SRI leads to severe serotonin toxicity in ~50% of cases (even with therapeutic doses).
If a patient is admitted to the hospital with serotonin toxicity after co-ingesting a MAOI and [S]SRI, transfer the patient to the ICU and consider consulting a toxicologist.
None of the TCAs are able to precipitate serotonin toxicity when given with MAOIs except for clomipramine and imipramine (do not combine these medications with MAOIs given their relatively potent serotonin reuptake activity).
While the treatment of serotonin toxicity is supportive (e.g., fluids, benzodiazepines, and active cooling), not understanding the spectrum concept or taking into consideration the specific medications ingested is ill-advised.
According to Professor Whyte & Dr. Gillman, serotonin toxicity and NMS are as similar as “chalk and cheese.”
References:
Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005 Mar 17;352(11):1112-20.
Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991 Jun;148(6):705-13.
Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003 Sep;96(9):635-42.
Evans RW, Tepper SJ, Shapiro RE, Sun-Edelstein C, Tietjen GE. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010 Jun;50(6):1089-99.
Foong AL, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol. 1999;13(1):100-9.
Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006 Mar;21(2):117-25.
Gillman PK. CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J Psychopharmacol. 2011;25(3):429-436.
Gillman PK. Successful treatment of serotonin syndrome with chlorpromazine. Med J Aust. 1996 Sep 16;165(6):345-6.
Gillman PK. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737-748.
Orlova Y, Rizzoli P, Loder E. Association of Coprescription of Triptan Antimigraine Drugs and Selective Serotonin Reuptake Inhibitor or Selective Norepinephrine Reuptake Inhibitor Antidepressants With Serotonin Syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
Rojas-Fernandez CH. Can 5-HT3 Antagonists Really Contribute to Serotonin Toxicity? A Call for Clarity and Pharmacological Law and Order. Drugs Real World Outcomes. 2014;1(1):3-5.